Chapter 8 Vascular Malformations
Vascular malformations (VMs) are developmental abnormalities of the vascular system. They should be differentiated from vascular tumors or hemangiomas, because they have different etiology, growth patterns, treatment and outcome. Malformations can involve any segment of the vascular tree: arteries, capillaries, veins, or lymphatics. High-flow arteriovenous malformations are associated with shunting of large amount of arterial blood into the venous system. These lesions can have alarming hemodynamic manifestations, such as venous engorgement, distal limb ischemia, and high output cardiac failure. Patients with low-flow venous malformations are seen most commonly at vascular clinics; most have a benign clinical course and require no special treatment. In one series the ratio of venous versus arteriovenous malformations was 4 : 1.1 Most VMs are mixed and some complex malformations like Klippel-Trenaunay syndrome or Parkes-Weber syndrome are associated with developmental abnormalities of other tissues, such as bone and soft tissue overgrowth or digital abnormalities.2,3
Historical Notes
Birthmarks and congenital deformities afflicting mankind have been described by historians and depicted by painters for centuries. Slowing of the heart rate after compression of a high-shunt congenital arteriovenous malformation was first described by Nicoladoni in 1875.4 This so-called bradycardia sign of arteriovenous fistulas was observed later by Branham in a patient with acquired arteriovenous fistula.5 Malan and Puglionisi6 presented a detailed classification of VMs (angiodysplasia), although the first practical guidelines for clinical classification and treatment were given by Szilagyi et al.7,8 Rutherford,9 summarized the state of the art of VMs. Of the multiple classifications published on VMs, the Hamburg classification, in a revised form, is the one used most frequently.9–12 Although surgical excision has been recommended for local lesions, it is selective catheterization and embolotherapy, as well as percutaneous sclerotherapy, with absolute alcohol and most recently with foam, that have changed the multidisciplinary management of VMs in the last decades.1,12–14
Definition of Vascular Malformations and Vascular Tumors
Vascular malformations are localized errors of angiogenic development, whereas hemangiomas are vascular tumors. Mulliken and Glowacki15–17 defined the endothelial characteristics and cell biology of VMs and vascular tumors. The term hemangioma should be reserved for vascular tumors alone; during the proliferative phase, they undergo growth and then undergo resolution. The proliferative phase occurs during the first year, and spontaneous involution of hemangiomas is observed in 95% by the age of 7 years. The female-to-male ratio is 5 : 1. Thirty percent of the hemangiomas are present at birth, and the rest develop within the first 3 months of life. Endothelial hyperplasia is evident on biopsy specimens obtained from hemangiomas; these cells grow in tissue culture. In the proliferative phase, they incorporate [3H]-thymidine and have an increased mast cell count.15 Most patients with hemangiomas require no treatment at all.
In contrast, VMs are developmental, congenital abnormalities. There is increasing evidence that aberrant signaling at the molecular level results in dysfunction of normal proliferation, differentiation, maturation, and apoptosis of the vascular cells.18–20 Localized superficial, mostly venous or capillary VMs (“birthmarks” of the skin and the mucosa) are the most frequent, but VMs also occur in the skeletal muscles, pelvis, chest, visceral organs such as the lungs, gastrointestinal system, and brain. The abnormal vascular channels are lined by a continuous endothelium and surrounded by abnormal complement of mural cells. Ninety percent of these cells are present at birth, and the male-to-female ratio is 1 : 1. VMs show no endothelial proliferation, no cell growth is observed in tissue culture, the cells do not incorporate [3H]-thymidine, and no mast cells have been observed in biopsy specimens.15–17 Clinically, no proliferation or spontaneous involution has been observed in VMs. The growth of the malformation is usually commensurate with the growth of the child, although hemodynamic factors (arteriovenous shunting, venous stasis) can accelerate growth and morbidity. Most low-flow VMs have a benign course, although complications such as bleeding, thrombophlebitis, skin changes, or infection may need treatment. High-flow arteriovenous malformations usually have a more ominous course and a worse prognosis. Treatment of these lesions is frequently needed.
Development of the Vascular System
The classification, clinical presentation, and prognosis of VMs are largely dependent on the point at which there is an arrest or abnormality in the development of the vascular system; therefore it is worthwhile to review briefly the normal development of the vascular tree of the limbs. Primitive vascular channels first appear in the third week of gestation. During its development, the vascular system undergoes differentiation through multiple stages.21 Stage 1 is the undifferentiated stage with only a capillary network being present. Stage 2 is the retiform stage when large plexiform structures can be seen. In stage 3, by the third week of gestation, the maturation stage includes development of large channels, arteries, and veins.
Vascular endothelial growth factor (VEGF) secreted by keratinocytes has been found to be responsible for inducing penetration of capillary vessels into the avascular epidermis.22 This invasion and the subsequent arterial differentiation are also guided by VEGF originating from sensory nerves.23 A defective migratory response of endothelial cells toVEGF is the consequence of abnormal signaling of VEGF receptors. Malformations develop if the differentiation is abnormal and there is an arrest in the development of normal vascular tissue. It is, indeed, the persistence of the normal embryonic vascular system and any additional abnormal development that result in VMs.
Classification
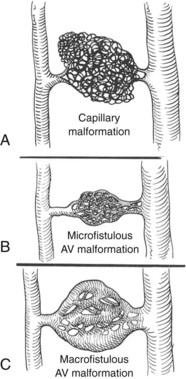
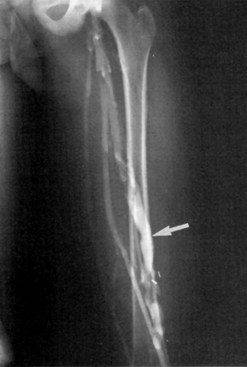
Classification has been difficult because of the complex presentation and frequently mixed nature of VMs. Malan and Puglionisi6 attempted to separate VMs based on anatomic appearance and the presence or absence of arteriovenous shunting. The classification by Szilagyi and colleagues7,8 was based primarily on Woollard’s stages of embryologic development: capillary malformations develop when there is an arrest in stage 1 (Figure 8-1). Although Szilagyi named them hemangiomas, these are not tumors, but capillary or cavernous VMs. Microfistulous or macrofistulous arteriovenous malformations develop if there is an arrest in stage 2 (see Figure 8-1). Persistence of large embryonic veins that develop in stage 3 is seen in patients with persistent sciatic vein (Figure 8-2) or in those with large lateral veins of the leg (Figure 8-3). There are many mixed VMs owing to involvement of several segments of the vascular system (capillaries, veins, lymphatics).
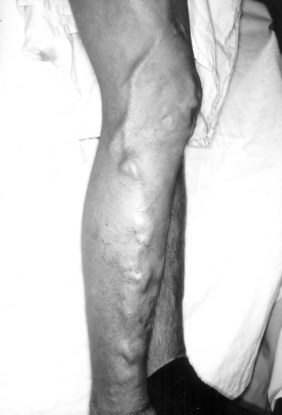
FIGURE 8-3 Persistent lateral embryonic veins in a 19-year-old male with Klippel-Trenaunay syndrome.
(From Noel AA, et al: Surgical treatment of venous malformations in Klippel-Trenaunay syndrome. J Vasc Surg 32:840–847, 2000.)
Forbes and colleagues24 distinguished VMs based on hemodynamic and contrast angiographic appearance. Depending on the amount of blood supplying the malformation, high-flow and low-flow lesions are distinguished. There are high-shunt and low-shunt lesions; the size of the shunts is determined by the volume of blood that enters the feeding vessels. High-shunt lesions correspond with macrofistulous arteriovenous malformations, whereas microfistulous arteriovenous malformations are low-shunt lesions. A whole spectrum of malformations can be found between these two extremes.
The most recent and widely used classification is the 1988 Hamburg classification, with modifications by Rutherford, Lee, and Gloviczki (Table 8-1).9–1225 Malformation is classified by the predominant vascular defect (e.g., arterial, venous, arteriovenous, capillary, lymphatic, and combined); it is further classified for most categories into truncular or extratruncular form depending on the involvement of major axial vessels or branches of major arteries or veins.
TABLE 8-1 Hamburg Classification of Vascular Malformations (Revised)
| Affected Segment of the Vascular System | Anatomic Forms |
|---|---|
| Arterial malformations | Truncular forms |
| Aplasia or obstruction | |
| Dilatation | |
| Extratruncular forms | |
| Infiltrating | |
| Limited | |
| Venous malformations | Truncular forms |
| Aplasia or obstruction | |
| Dilatation | |
| Extratruncular forms | |
| Infiltrating | |
| Limited | |
| Arteriovenous malformations (with shunting ) | Truncular forms Deep AV fistula Superficial AV fistula Extratruncular forms Infiltrating Limited |
| Capillary malformations | |
| Lymphatic malformations | Truncular forms |
| Aplasia or obstruction | |
| Dilatation | |
| Extratruncular forms | |
| Infiltrating | |
| Limited | |
| Combined vascular malformations | Truncular forms Arterial and venous Hemolymphatic Extratruncular forms Infiltrating hemolymphatic |
| Limited hemolymphatic |
AV, Arteriovenous.
The truncular forms of arterial VMs include aplasia or obstruction, stenosis, coarctation, dilation, or aneurysms. These forms include malformations such as the persistent sciatic artery or an aberrant left subclavian artery that runs behind the esophagus and causes the typical syndrome of dysphagia lusoria.26 Thoracic or abdominal aortic coarctation (Figure 8-4),27,28 anomalies of the aortic arch or persistence of embryonic mesenteric vessels, are additional examples of these malformations. The extratruncular forms can be diffuse or localized.
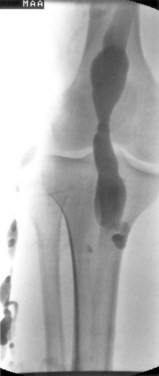
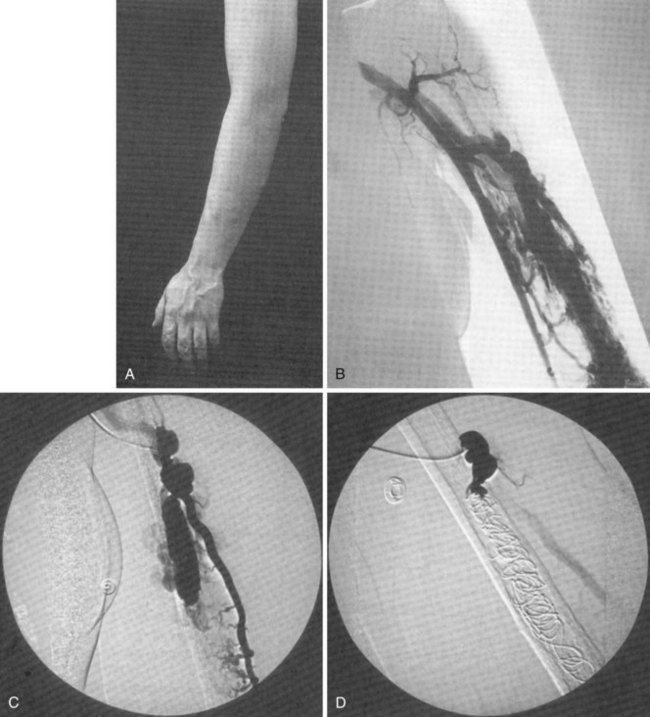
Venous malformations may also be truncular; these include aplasia or obstruction, stenosis or hypoplasia, dilations, and aneurysms. Many patients with Klippel-Trenaunay syndrome have persistence of large embryonic veins or hypoplasia, dilation or aneurysmal dilatation of the deep veins of the limb (Figure 8-5).29,30 The estimated prevalence of deep venous anomalies in patients with predominantly venous malformations was 47% in one study.31 Phlebectasia was the most frequent (36%), followed by aplasia or hypoplasia of the deep venous trunks (8%) and venous aneurysms (8%).
Extratruncular venous VMs are the most frequent malformations, and they can be diffuse (Figure 8-6; see color plate) and localized. Most venous malformations are localized defects of vascular morphogenesis that manifest as single or multiple bluish-purple lesions, mainly in the skin and the mucosa.32 Biopsy specimens show enlarged endothelial-lined veinlike channels with abnormal smooth-muscle cells.
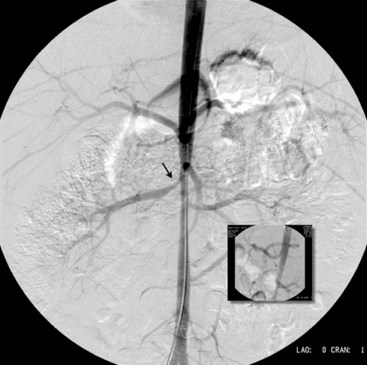
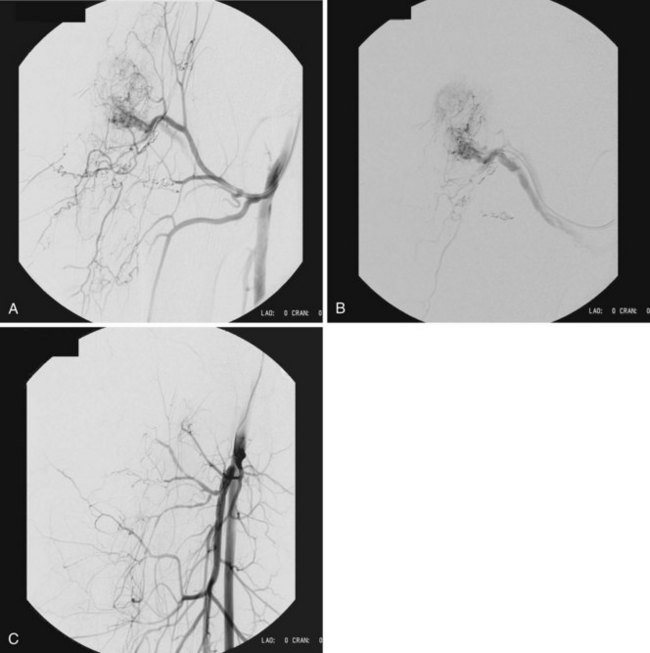
Arteriovenous malformations are divided into truncular and extratruncular lesions, both can be localized and diffuse. Lesions with clinical or angiographic evidence of arteriovenous communications on the limbs (Parkes-Weber–type malformations) or pelvis are those most frequently seen by a vascular surgeon. Low shunt (Figure 8-7) and high shunt malformations (Figure 8-8) are distinguished. The hereditary arteriovenous VMs in the lung and sometimes in the brain and gut are part of hereditary hemorrhagic teleangiectasia (HHT) syndrome.32 Arteriovenous malformations are perhaps most frequent in the central nervous system, and vascular surgeons performing carotid arteriography and stenting should recognize and be familiar with arteriovenous VMs and high-flow arteriovenous fistulas of the brain.
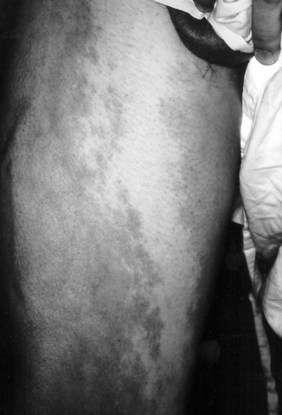
Capillary malformations, or port-wine stains, are frequent. These cutaneous lesions appear as a red macular stain that darkens over years (Figure 8-9). Capillary malformations are typical in patients with Sturge-Weber syndrome, Klippel-Trenaunay syndrome, and Parkes Weber syndrome.3,12
Lymphatic malformations have been found frequently in some series. The truncular form includes obstruction or hypoplasia causing congenital lymphedema,33,34 or dilation leading to valvular incompetence and rupture of lymphatics causing chylous effusions or chylocutaneous fistulas owing to reflux of the chyle.35,36 Most lymphatic cysts and lymphangiomas are lymphatic malformations, and many venous malformations contain lymphatic tissue.
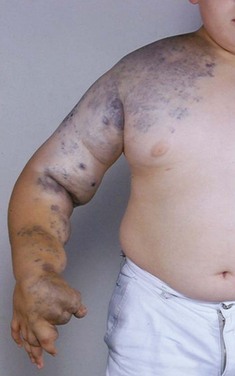
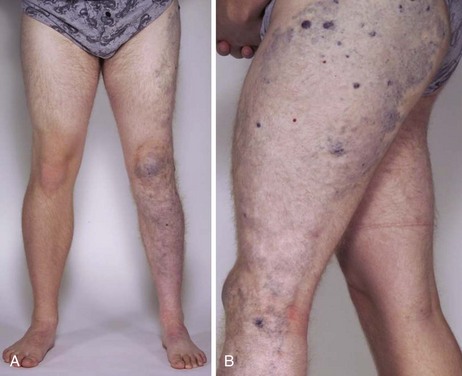
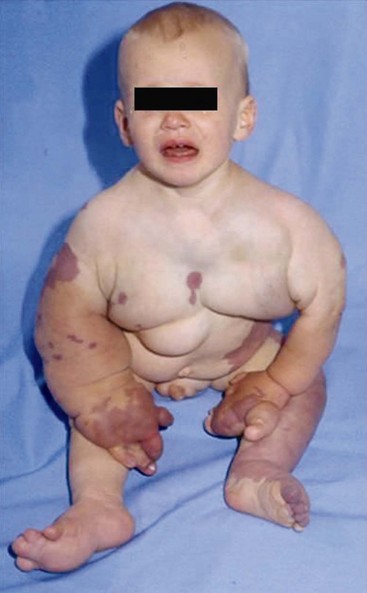
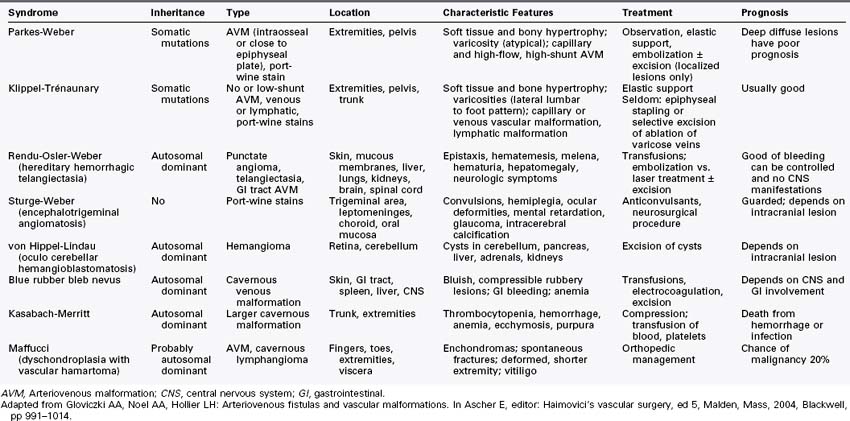
More than 70% of the VMs are mixed, and these frequently complex abnormalities can include arterial, capillary, venous, or lymphatic elements as well. Although the Hamburg classification discourages the use of eponyms, some terms are named after physicians who first described the conditions; these have been widely accepted and used. The list of clinical syndromes of vascular malformation includes Parkes-Weber, Klippel Trenaunay, Servelle-Martorell (Figure 8-10; see color plate), Sturge-Weber, Rendu-Osler-Weber, von Hippel-Lindau, Kasabach-Merritt, Proteus (Figure 8-11; see color plate), and Maffucci syndromes (Table 8-2.)12,13,30,37,38
Genetics
Genetic information on VMs has greatly increased in recent years.39 Most VMs are sporadic, but autosomal dominant inheritance has also been described. Genetic studies of families have resulted in the identification of mutated genes18,40–42 that have an important role in angiogenesis. These mutated genes in some patients encode tyrosine kinase receptors and intracellular signaling molecules.18 Vikkula and colleagues18 identified the endothelial-specific angiopoietin receptor TIE2/TEK, located on 9p21, as the cause of familial mucocutaneous VMs. Glomuvenous malformations (venous malformations with glomus cells, or glomangiomas) are similar to VMs; most of these lesions are inherited, and Boon and colleagues43 identified the gene glomulin, a novel locus on the short arm of chromosome 1.43
Port-wine stains have also been observed in families, and a genetic susceptibility for capillary malformations was suggested. Eerola and colleagues40 identified a large locus, CMC1, on chromosome 5q. These authors used genetic fine mapping to identify a positional candidate gene, RASA1; heterozygous inactivating RASA1 mutations were detected in families manifesting capillary malformations. Of interest, arteriovenous malformation, arteriovenous fistula, or Parkes-Weber syndrome were also documented in all the families with this mutation.
Arteriovenous malformations in the lungs, brain, or gut may be part of hereditary hemorrhagic telangiectasia (HHT).44 Two genes encoding transforming growth factor beta receptor–associated proteins have been identified causing HHT1 and HHT2.
Primary congenital lymphedema can be hereditary (Milroy disease), whereas late onset primary lymphedema was also observed in multiple members of the same families (Meige disease).45,46 Congenital lymphedema has been linked to chromosome 5q35.3, where the VEGFR3 gene (vascular endothelial growth factor receptor 3) is located. It is likely that congenital lymphedema is caused by lack of sufficient signaling via the VEGFR3 receptor.18
Incidence
VMs occur in approximately 1.5% of the population.31 Published series of VMs seen at referral centers suggest that predominantly venous malformations are the most common vascular anomalies; they are estimated to occur in between 1/5000 and 1/10,000 childbirths.18 Venous malformations are certainly the most frequent that require medical attention.13 Still, it is likely that capillary malformations or port-wine stains of the skin and mucosa are the most frequent VMs, occurring in 0.3% of childbirths.40 Among 797 patients with VMs reported by Lee and colleagues,1 40% had predominantly lymphatic malformations. Arteriovenous shunts in this series occurred in 9.5%.
Clinical Presentations
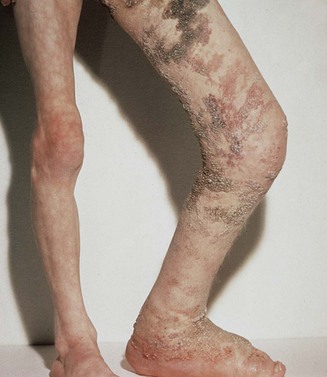
Patients with VMs are frequently asymptomatic, and a birthmark of the skin or the mucosa is a cosmetic deformity only. However, patients who seek consultations by vascular surgeons may have a complex presentation or juvenile varicosity, and the limbs or pelvis may have extensive involvement by the malformation. Clinical presentations include varicose veins (see Figure 8-3), limb edema or overgrowth (Figure 8-12; see color plate), port-wine stain or digital anomalies (Figures 8-13, 8-14; see color plate). The affected limb or pelvis may harbor a mass that is pulsatile and may have a systolic-diastolic bruit and a palpable thrill. The varicose veins are usually atypical, lateral or suprapubic, although occasionally varicosity may involve the great saphenous vein and its tributaries. Bleeding or leakage of lymph fluid from VMs is not infrequent. Thrombophlebitis, cellulitis and lymphangitis, skin lesions, induration, pigmentation, and ulcerations can be signs of chronic venous insufficiency. Many of the patients with mixed lesions have associated lymphedema. Patients with pelvic involvement may exhibit hematuria and rectal bleeding. Any patient with varicose veins or port-wine stains with a longer or shorter limb could also have an underlying vascular malformation. Patients with primary or secondary lymphedema have limbs of identical length.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree