, Jay S. Giri2, Joseph M. Garasic3 and Joseph M. Garasic4
(1)
Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA
(2)
Peripheral Intervention, Cardiology Division, Hospital of the University of Pennsylvania, Philadelphia, PA, USA
(3)
Harvard Medical School, Boston, USA
(4)
Peripheral Vascular Intervention, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
Peripheral artery disease (PAD) is common and often unrecognized. Patients with PAD are at high-risk for adverse cardiovascular events. Management of lower extremity PAD has two components: aggressive treatment of cardiovascular risk factors as well as treatment of lower extremity symptoms. In some cases of carotid and renal artery disease, interventional treatment is performed in asymptomatic lesions with hopes of altering the natural disease history and reducing future events.
Venous thromboembolism is common and can present as deep venous thrombosis (DVT) or pulmonary embolism (PE). Both have high morbidity and present management challenges in a wide range of clinical circumstances.
Abbreviations
ABI
Ankle-brachial index
ACE
Angiotensin converting enzyme
ARB
Angiotensin II receptor blocker
ASA
Aspirin
CAS
Carotid artery stenting
CEA
Carotid endarterectomy
CLI
Critical limb ischemia
CTA
Computed tomographic angiography
CV
Cardiovascular
DVT
Deep venous thrombosis
FMD
Fibromuscular dysplasia
HTN
Hypertension
MI
Myocardial infarction
MRI
Magnetic resonance imaging
NSTEMI
Non-ST-elevation myocardial infarction
PAD
Peripheral artery disease
PE
Pulmonary emoblism
RBBB
Right bundle branch block
RVSP
Right ventricular systolic pressure
Introduction
Peripheral artery disease (PAD) is common and often unrecognized. Patients with PAD are at high-risk for adverse cardiovascular events. Management of lower extremity PAD has two components: aggressive treatment of cardiovascular risk factors as well as treatment of lower extremity symptoms. In some cases of carotid and renal artery disease, interventional treatment is performed in asymptomatic lesions with hopes of altering the natural disease history and reducing future events.
Venous thromboembolism is common and can present as deep venous thrombosis (DVT) or pulmonary embolism (PE). Both have high morbidity and present management challenges in a wide range of clinical circumstances.
Peripheral Artery Disease (PAD)
Prevalence
∼4 % in patients over age 40
Increases with age and cardiovascular (CV) risk factors
∼15–30 % in patients over age 70
CV Implications [1]
Roughly 50 % of PAD patients will have coronary artery disease (CAD)
CV events are more common than ischemic limb events
Ankle-brachial index (ABI) <0.7 = risk of myocardial infarction (MI) is 20 % at 5 years (double the highest-risk Framingham group)
ABI 0.7–.09 = risk of MI is 10 % at 5 years
Patients at risk for PAD (All of the below risk groups have pre-test probabilities of over 15 % and should be screened for PAD) (Table 10-1)
Table 10-1
Groups with high PAD prevalence
Known atherosclerotic coronary, carotid, or renal artery disease
Age >70
Age >50 with DM or smoking
Age <50 with DM and an additional risk factor (smoking, hypertension, hyperlipidemia)
Abnormal LE pulse examination
Exertional leg symptoms
Presentation (Fig. 10-1)
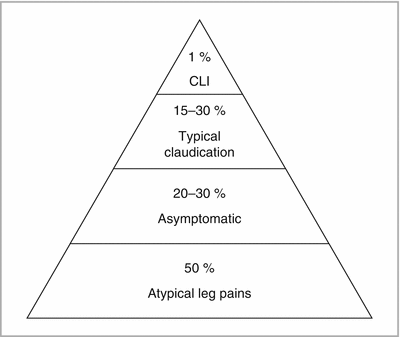
FIGURE 10-1
Presentation of PAD
Symptoms
Typical claudication – cramping calf pain exacerbated by exertion and relieved by rest
Critical limb ischemia (CLI) – pain at rest in the foot, non healing ulcer, gangrene
Physical examination
Poor peripheral pulses (femoral, popliteal, pedal)
Femoral artery bruit on auscultation
Elevation pallor (foot develops pallor when raised)
Dependent rubor (foot slowly becomes red when returned to the ground)
Poor capillary refill (>3 s)
Ulcerations on the toes, intertriginous spaces, borders of the feet
Testing
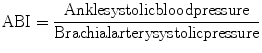
ABI Interpretation
1.40
Uninterpretable/incompressible
1.00–1.39
Normal
0.91–099
Borderline
0.71–0.90
Mild PAD
0.41–0.70
Moderate PAD
<0.40
Severe PAD
Uninterpretable ABI due to incompressible vessels secondary to Mockenburg’s medial artery calcification.
This is more common in diabetic and elderly populations.
Toe brachial index <0.7 is sensitive for dx of PAD in settings of uninterpretable/incompressible ABI (Table 10-2).
Table 10-2
Diagnostic tests for PAD evaluation
Pros
Cons
ABI
Non-invasive, fastest, office-based
No clarity regarding level of disease
Segmental pressures with pulse volume recordings
Non-invasive, rapid, no contrast
Does not clarify anatomic details
Arterial ultrasound
Non-invasive, no contrast
Operator-dependent, may not be able to image suprainguinal and/or infrapopliteal vessels, time consuming
CTA
Non-invasive
IV contrast, radiation, difficult to interpret in the setting of heavy vascular calcification
MRA
Non-invasive
Gadolinium, expensive, may overestimate stenosis severity
Digital subtraction angiography (DSA)
Best quality anatomic information, option for concurrent therapeutic procedure
Invasive, IV contrast, technical expertise necessary
Medical Management of PAD [2]
Treat DM to glycohemoglobin <7 %
Daily Foot Care and Regular Podiatry Appointments
Smoking Cessation
Lipid Lowering Therapies – Statins decrease CV events and may improve leg functioning (i.e.: pain-free walking distance)
Treatment of hypertension to guideline derived goals.
ACE-inhibitors may improve leg functioning
Beta-blockers are NOT harmful
Anti-platelet therapy
Coumadin – Coumadin is not recommended in addition to anti-platelet therapy for PAD [5]
Symptomatic Medical Therapy
Supervised exercise rehabilitation improves pain-free walking distance.
Increased daily activity leads to decreased mortality
3–6 months of cilostazol is recommended (contraindicated in patients with heart failure)
Interventional Therapy
Contraindications
Lack of symptoms
Lack of pressure gradient across an angiographic stenosis
Endovascular revascularization
Indicated if life or work-limiting symptoms exist after a trial of medical and exercise therapy
Primary stenting should not be performed in the femoropopliteal segments (i.e.: use stents for failure of angioplasty or atherectomy techniques)
Surgical revascularization
Indicated if life or work-limiting symptoms exist after a trial of medical and exercise therapy and if not a good anatomic candidate for endovascular approach
Autogenous vein grafts are preferred to prosthetic grafts for lower extremity bypass due to improved long-term patency
Special topic: Critical Limb Ischemia (CLI)
Revascularize suprainguinal segments first for treatment of CLI
If ulcer persists, consider revascularization of infrainguinal segments with the goal of establishing “straight-line” blood flow to the foot
Open repair and endovascular repair of the lower extremities had equivalent results in the BASIL trial [6]
Special topic: Acute Limb Ischemia (ALI)
Initiate parenteral anticoagulation
Seek consultation from an expert vascular interventionalist or surgeon
If ALI is less than 14 days, catheter-directed thrombolysis with or without adjunctive catheter-based mechanical therapies may be used to restore vessel patency
The decision between open surgical and endovascular treatment is influenced by the likelihood of the technical success and the rapidity of revascularization with each strategy.
Renovascular Disease
Atherosclerotic Renal Artery Stenosis
Prevalence
Approaches 10 % in consecutive patients undergoing cardiac catheterization
May be up to 20 % in patients with a history of diabetes mellitus and hypertension
Presentation
Resistant hypertension
Worsening renal function with addition of ACEi/ARB (most common in bilateral disease)
Acute pulmonary edema (in bilateral disease)
Physical exam – severe hypertension, abdominal or flank bruits, volume overload (in bilateral disease)
Imaging Studies
Doppler ultrasound – low cost, non-invasive, no iodinated contrast but sensitivity is highly operator-dependent< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


