, Gaurav A. Upadhyay1, Henry Gewirtz2 and Henry Gewirtz3
(1)
Harvard Medical School Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School, Boston, USA
(3)
Nuclear Cardiology, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
Exercise stress testing and radionuclide myocardial perfusion imaging (MPI) are commonly performed in order to provide medical diagnosis of coronary artery disease (CAD) and to assist with risk stratification and clinical management for patients with established CAD. Exercise stress testing also provides an objective, standardized measure of the patient’s functional capacity, which is important prognostically and also an essential component of any cardiac rehabilitation program.
MPI is performed in the context of stress testing determine the presence and extent of myocardial ischemia. Either treadmill exercise or a pharmacologic ‘stress’ with adenosine, regadenoson, or dobutamine is employed. Next, an objective method is utilized to assess the degree of ischemia induced. Commonly used single-photon emission computed tomography (SPECT) MPI tracers include technetium-99m-sestamibi or tetrofosmin, and in some labs thallium-201, for rest imaging. Both of these determine relative regional myocardial flow in order to assess for ischemia. However, positron emission tomography (PET) can assess absolute regional myocardial flow and the availability of new tracers for PET will greatly expand the use of PET for MPI.
This chapter presents a general frame work for MPI stress test selection and also reviews American College of Cardiology (ACC)/American Heart Association (AHA)/American Society of Nuclear Cardiology (ASNC) guidelines and appropriateness criteria regarding indications for such testing.
Abbreviations
ACC
American College of Cardiology
AHA
American Heart Association
Bq
Becquerel
11C
Carbon
CABG
Coronary artery bypass graft surgery
CAD
Coronary artery disease
Ci
Curie
CKD
Chronic Kidney disease
COPD
Chronic obstructive pulmonary disease
CORE
Center of Rotation Error
DBP
Diastolic blood pressure
18F
Fluorine
ECG
Electrocardiogram
EF
Ejection fraction
G
Gray
HR
Heart rate
IC
Internal conversion
IT
Isomeric transition
IVCD
Intraventricular conduction delay
LAD
Left anterior descending
LBBB
Left bundle branch block
LMCA
Left main coronary artery
LV
Left ventricular
LVEF
Left ventricular ejection fraction
MET
Metabolic equivalents of task
MI
Myocardial infarction
mph
Miles per hour
99Mo
Molybdenum
MPI
Myocardial perfusion imaging
13N
Nitrogen
15O
Oxygen
PET
Positron emission tomography
PVC
Premature ventricular complexes
R
Roentgen
RAD
Radiation absorbed dose
82Rb
Rubidium
RMR
Resting metabolic rate
SBP
Systolic blood pressure
SDS
Summed Difference Score
SPECT
Single-photon emission computed tomography
SRS
Summed Rest Score
SSS
Summed Stress Score
ST60/ST80
ST-segment is assessed at the J-point and 60/80 ms
Sv
Sievert
99mTc
Technetium-99m
201TI
Thallium-201
TID
Transit ischemic dilatation
VO2
Estimated oxygen uptake
Introduction
Exercise stress testing and radionuclide myocardial perfusion imaging (MPI) are commonly performed in order to provide medical diagnosis of coronary artery disease (CAD) and to assist with risk stratification and clinical management for patients with established CAD. Exercise stress testing also provides an objective, standardized measure of the patient’s functional capacity, which is important prognostically and also an essential component of any cardiac rehabilitation program.
MPI is performed in the context of stress testing to determine the presence and extent of myocardial ischemia. Either treadmill exercise or a pharmacologic ‘stress’ with adenosine, regadenoson, or dobutamine is employed. Next, an objective method is utilized to assess the degree of ischemia induced. Commonly used single-photon emission computed tomography (SPECT) MPI tracers include technetium-99m-sestamibi or tetrofosmin, and in some labs thallium-201, for rest imaging. All of these determine relative regional myocardial flow in order to assess for ischemia. However, positron emission tomography (PET) can assess absolute regional myocardial flow and the availability of new tracers for PET will greatly expand the use of PET for MPI.
This chapter presents a general frame work for MPI stress test selection and also reviews American College of Cardiology (ACC)/American Heart Association (AHA)/American Society of Nuclear Cardiology (ASNC) guidelines and appropriateness criteria regarding indications for such testing.
Physics, Radiation Safety, and Instrumentation
A basic background in nuclear physics and radiation is essential for better understanding of nuclear cardiology, and in order to provide protection for oneself and others from radiation.
A.
Basic nuclear physics
Background: Radionuclides commonly used for SPECT myocardial perfusion imaging include technetium-99m ( 99m Tc) and thallium-201 ( 201 TI). Positron emission tomography (PET) utilizes Rubidium (82Rb), oxygen (15O), nitrogen (13N), carbon (11C), and fluorine (18F) to label a wide variety of tracer molecules in order to assess myocardial blood flow and metabolism. In addition, PET can reveal important information about molecular signaling and responses of the myocardium to pathological states as ischemia and heart failure (HF). In sharp contrast to SPECT imaging, which can assess relative differences in tracer distribution, PET is capable of absolute quantitative measurement of myocardial tracer content. Thus PET can differentiate between normal and abnormal regions of the heart with better accuracy and allows direct quantitative comparison between patients.
Commonly used tracers (Table 8-1)
Table 8-1
Tracers commonly used for myocardial perfusion imaging
Radionuclides
Generation
Half-life
Gamma rays/x-rays (keV)
Myocardial Extraction fraction (%)
Comments
SPECT
Technetium-99m ( 99m Tc)-Sestamibi
On-site generator
6 h
140 (ideal photopeak)
60
Most commonly used for medical procedures
Thallium-201 ( 201 TI)
Cyclotron
73 h
80
75
Active, Na/K ATPase-dependent
PET
Rubidium (82Rb)
Cyclotron
76 s
511
60
Greater spatial and temporal resolution compared with SPECT
Absolute quantization of myocardial blood flow possible
B.
Radiation Exposure, Units, and Dose Limits
Radiation exposure
In the United States, ionizing radiation from medical procedures make up almost 50 % of radiation exposure, while in other parts of the world, natural background comprises the majority of radiation exposure.
The linear no-threshold model: there is a linear dose response relationship in future risk of cancer but any exposure to ionizing radiation, can induce a future risk of malignancy.
Units of radiation dose
Radiation exposure: ionizing radiation concentration in air, measured in Roentgen (R)
Absorbed dose: how much is absorbed in a specific tissue, measured in radiation absorbed dose (RAD) or Gray (Gy). Gy = 100 RAD
Effective dose: equivalent whole body dose taking into consideration the organ irradiated, usually used in assessing risk of radiation, measured in Sievert (Sv) or radiation equivalent dose (REM). Sv = 100 REM
Average effective radiation dose in common cardiac procedures (Table 8-2)
Procedure
Average effective dose (mSv)
CXR, posteroanterior
0.02
CXR, posteroanterior and lateral
0.1
CT coronary calcium score
3
Coronary angiogram
7
CT chest
8
Nuclear cardiac stress test (Rest/Stress Tc-99m-MIBI exam)
15–20
Cardiac (dose is tracer and protocol dependent:stress only 30 mCi 13-N-ammonia)
2.5
CTA, pulmonary embolism protocol
15
Coronary angioplasty or stent
15
CT coronary angiogram (with current 128 slice CT and dedicated coronary protocol)
2–5
Radiation Dose Limits
Guiding principle: ALARA – As Low As Reasonably Achievable.
Three factors to achieve this goal
Decrease time.
Increase distance: Radiation exposure diminishes in an inverse square relative to distance from radiation source.
Use shielding
Non-occupational dose limit: 5 mSv per year
Occupational dose limit: 50 mSv per year
Stress Testing and Protocols
Induction of Myocardial Ischemia
Exercise
Types of exercise testing: commonly treadmill or bicycle. Arm ergometry or rowing machine available.
Subject preparation
Medications:
Diagnosis of CAD: negative inotropic medications, particularly β-blockers are usually held so that a maximal heart rate (HR) response may be achieved.
Known CAD, ischemic threshold or efficacy of antianginal therapy: take usual cardiovascular medications
Exercise protocols
Standard Bruce Protocol: the most widely used and validated protocol. A multi-stage test in which successive stages increase estimated oxygen uptake (VO2) and myocardial demand [1]
Stage I is roughly equivalent to four metabolic equivalents of task
Modified Bruce Protocol : in assessment of patients soon after myocardial infarction (MI) or in those who are elderly or sedentary [2]. The protocol adds two stages prior to Stage I of the Standard Bruce Protocol:
Other protocols include the Cornell, Naughton and Balke protocols, all of which offer the ability to begin at a lower workload and assess at more stages.
Test termination and adequacy
Test termination: based on perceived exertion, anginal symptoms, electrocardiogram (ECG) assessment, and clinical symptoms [3]
Absolute indications for terminating exercise testing include (from the 2001 AHA Exercise Standards for Testing and Training) [3]:
ST-segment elevation (>1.0 mm) in leads without diagnostic Q-waves
Drop in systolic blood pressure (SBP) of >10 mmHg from baseline, despite an increase in workload, when accompanied by other evidence of ischemia
Moderate-to-severe angina that is intolerable
Central nervous system symptoms (e.g., ataxia, dizziness, or
near-syncope)
Signs of poor perfusion (cyanosis or pallor)
Sustained ventricular tachycardia (VT)
Technical difficulties in monitoring ECG or SBP
Subject’s desire to stop
Relative indications for terminating exercise testing [3] include:
ST or QRS changes such as excessive ST depression (>2 mm of horizontal or downsloping ST-segment depression) or marked axis shift
Drop in SBP of >10 mmHg from baseline, despite an increase in workload, in the absence of other evidence of ischemia
Increasing chest pain
Fatigue, shortness of breath, wheezing, leg cramps, or claudication
Arrhythmias in addition to sustained ventricular tachycardia, others, such as multifocal premature ventricular complexes (PVC), triplets of PVCs, supraventricular tachycardia, heart block, or bradyarrhythmias
General appearance of exhaustion or poor tissue perfusion
Hypertensive response (SBP > 250 mmHg or diastolic blood pressure (DBP) > than 115 mmHg)
Development of bundle-branch block or intraventricular conduction delay (IVCD) that cannot be distinguished from VT
Adequate if the patient can achieve at least 85 % of her or his maximal predicted HR [4]
Maximal predicted HR = 220 – age (in years)
Peak double product or rate pressure product (RPP) = HR×SBP
RPP of apparently healthy males (n > 700; ages 25–54) was between 25,000 (10th percentile) and 40,000 (90th percentile) [3]
Contraindications to exercise stress testing (Table 8-3)
Table 8-3
Contraindications to exercise testing
Absolute
Acute MI (within 2 days)
High-risk unstable angina
Uncontrolled cardiac arrhythmias
Severe aortic stenosis
Uncontrolled symptomatic HF
Acute pulmonary embolus or pulmonary infarction
Acute aortic dissection
Acute myocarditis or pericarditis
Unstable patient for non-cardiac reason
Patient is unable to give consent
Relative
Left main coronary stenosis
Moderate stenotic valvular heart disease
Electrolyte abnormalities
Severe arterial hypertension
Significant arrhythmias
Hypertrophic cardiomyopathy and other forms of outflow tract obstruction
High-degree atrioventricular block
Mental or physical impairment leading to inability to exercise adequately
Exercise stress testing is associated with a small risk of death (0.5 per 10,000), MI (3.6 per 10,000) and serious arrhythmia (4.8 per 10,000) [3].
When the patient is unable to exercise or ECG will be uninterpretable for ischemia (e.g.; LBBB, LVH, WPW), pharmacological stress testing is employed. In patients unable to exercise long term prognosis may be somewhat worse, due to the fact that inability to exercise is a marker of poorer functional status [5].
Vasodilators
Background: induce coronary vasodilatation and hyperemia. Regional flow differences between diseased (less hyperemic) and normal vessels (more hyperemic) are visualized as perfusion defects using MPI
Vasodilator stress agents: adenosine, regadenoson and dypridamole are the most commonly used agents in the US [6],
Adenosine: is an endogenously present purine nucleoside molecule which produces dose-dependent myocardial hyperemia through activation of adenonsine A2A receptors in the coronary vasculature [7]
Mechanism: nonselective agonist of A1, A2A, A2B, A3, and A4 receptors (throughout the body) with half-life of < 10 s
Side effects: flushing, headache, nausea, mild fall in arterial blood pressure (BP), bradyarrhythmia, bronchospasm, and transient A-V block including complete heart block
Protocol: IV adenosine at140 μg/kg/min for 5–6 min. Radiotracer is injected after 2–3 min of infusion.
Regadenoson: recently approved selective A2A agonist [8]
Mechanism: high affinity selective A2A agonism, peak of 1–4 min and half-life of 30 min
Side effects: similar to adenosine but at lower overall incidence than for adenosine.
Protocol: Regadenoson is administered as a single bolus of a pre-filled intravenous syringe of 0.4 mg (in 5 mL) over 10 s. The radiotracer is subsequently injected before the end of the first minute after infusion
Dipyridamole: produces coronary hyperemia by impairing the cellular reuptake of adenosine [9]
Mechanism: inhibits nucleoside transporter and causes an accumulation of adenosine in the interstitial space. It has a substantially longer half-life than adenosine or regadenoson.
Side effects: similar to those of adenosine and regadenoson, but usually longer lasting
Protocol: infused at 0.56 mg//kg over 4 min. The radionuclide tracer is injected 7–9 min after initiation. Aminophylline is also required 1–2 min after radiotracer in order to reverse effects
Subject preparation
Medications: aminophylline is used as an antidote to adenosine, regadenoson, and dipyridamole, and cannot be used within the 24 h preceding the test with vasodilator compounds (Table 8-4)
Table 8-4
Contraindications for pharmacological stress testing
Adenosine/Regadenoson/Dipyridamole
Dobutamine
Severe asthma, chronic obstructive pulmonary disease (relative contraindication for regadenoson)
Unstable angina
SBP < 90
Recent MI (within 1–3 days)
HR < 40 bpm
SBP < 90
Xanthines: e.g., caffeine, tea, dark chocolate, aminophylline or theophylline within 24 h of testing
SBP > 200
High degree atrioventricular block/sick sinus syndrome
Significant history of ventricular tachyarrhythmias
Less than 2 days after MI
Severe aortic stenosis
Hypertrophic cardiomyopathy
Large aortic aneurysm or aortic dissection
Contraindications: patients at a greater chance for suffering the known side effects to vasodilator agents (Table 8-4)
Dobutamine
Background: inotropic/chronotropic stimulation with synthetic catecholamine, when exercise or vasodilator-stressing is not feasible or is contraindicated [10].
Mechanism: β-1 adrenoreceptor agonist (increase inotropy and chronotropy) but also with β-2 effects (can cause hypotension). Atropine can also be added to achieve target HR.
Side effects: arrhythmia is common. Nonsustained VT and sustained atrial fibrillation are not uncommon among patients with history of left ventricular dysfunction. Hypotension, angina, nonspecific chest pain, and dyspnea also occur in a minority of patients. Headache, paresthesias, and nausea have also been reported
Protocol: dobutamine infusion is escalated in scheduled doses, beginning with 5–10 μg/kg per min, up to 40 μg/kg with attention to maximal HR. Indications to terminate dobutamine infusion are similar as to those for exercise stress testing.
Subject preparation: All β-blocking medications must be held for a 24–48 h period
Contraindications: in patients in whom enhanced contractility, increased dP/dT are harmful and patients who are at high risk of side effects (Table 8-4)
Detection of Myocardial Ischemia
ElectrocardiogramGet Clinical Tree app for offline access
Background: a broad spectrum of ECG changes during exercise are considered normal. Myocardial ischemia, in particular, manifests on the ECG stress testing with ST segment changes
Physiologic ECG changes with stress [3]
P-wave: magnitude increases, particularly in the inferior leads
PR segment: shortens and slopes downward, particularly in the inferior leads. The ‘Ta wave,’ or repolarization of the atrial p-wave is thought to drive the downward sloping of the PR segment
QRS complex: a decrease in the R-wave amplitude is noted in the apical leads (V5, V6), associated with an increasing S-wave depth
J-point depression: The J-point (or J-junction) is depressed during exercise, particularly in the apicolateral leads (V4-V6). This is felt to be due to atrial repolarization (i.e., the ‘Ta’ wave) and often leads to ‘upsloping’ ST depressions of <2 mm
T-wave: decrease in T-wave amplitude seen in early in exercise, resolves within 1 min into recovery
Baseline ECG abnormalities which make it difficult to diagnose ischemia from ECG (Table 8-5)
Table 8-5
Baseline ECG abnormalities which make it difficult to diagnose ischemia from the electrocardiogram
Complete LBBB
Preexcitation syndrome
Left ventricular hypertrophy
Digoxin therapy
Greater than 1 mm of resting ST-segment depression
Electronically paced ventricular rhythm
Abnormal ECG responses during stress testing (See Fig. 8-1)
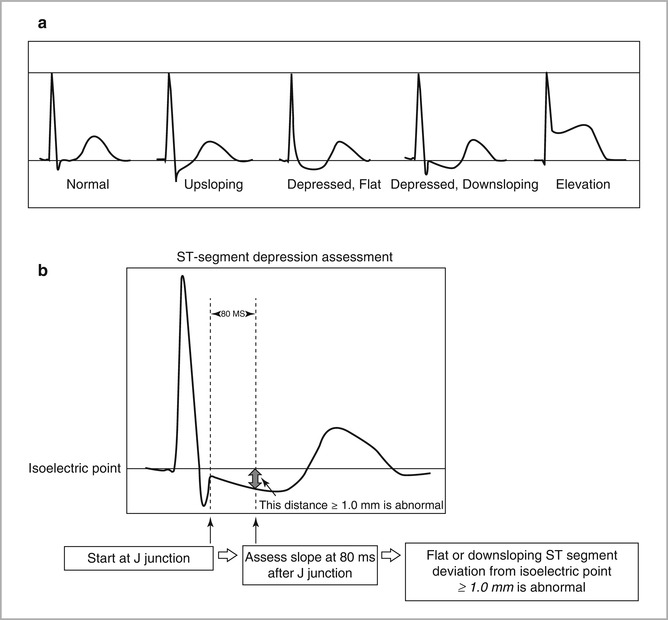
Figure 8-1
Abnormal and borderline ECG changes during exercise stress testing (Courtesy of Dr. Hanna Gaggin) (a) ST Segment Patterns (b) Measurement of ST Segment Depression
ST-segment depression: single most common manifestation of myocardial ischemia
Measured relative to the P-R segment, which serves as the isoelectric baseline for practical purposes (true isoelectric point is T-P segment)< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


