Chapter 57
Varicose Veins
Surgical Treatment
Mark D. Iafrati, Thomas F. O’Donnell Jr.,
Venous disease has received relatively little attention in the public arena and in vascular training programs, despite the fact that it is more prevalent in the United States than coronary artery disease, peripheral arterial disease, congestive heart failure, and stroke combined.1 The era of vascular intervention for varicose veins was ushered in by Friedrich Trendelenburg, in the 1860s, who not only popularized his eponymous Trendelenburg test for saphenous reflux but also performed great saphenous vein (GSV) ligation by making a transverse upper thigh incision to ligate and divide the proximal GSV.2 William Moore, an Australian surgeon, moved the site of ligation cephalad to the saphenofemoral junction. He was also the first to perform ambulatory venous surgery under cocaine anesthesia.3 Ligation of the saphenofemoral junction as it is practiced today was first described by John Homans in his seminal paper in 1916.4 The Mayo Brothers, postulating that there would be additional benefit in removing the saphenous vein, pursued excision of the GSV through an incision extending from the groin to below the knee.5 Effective at accomplishing its goal of eliminating GSV reflux, this maximally invasive approach required considerable operative time and resulted in significant wound complications. This extirpative technique was initially improved by the use of an external “ring vein enucleator.”6 The final technologic leap was introduction of the intraluminal stripper by Babcock.7 The latter half of the 20th century saw dramatic advances in diagnostic testing; however, surgical treatment of varicose veins benefited from only modest refinements after this early flurry of innovation.
The 21st century has begun with a resurgence of interest and innovation in venous disease. Increased awareness of thrombotic and venous reflux disease has been coupled with advances in the diagnosis and treatment of acute deep venous thrombosis (DVT) (see Chapter 51), and thermal ablation techniques for the treatment of chronic venous disease have proved appealing to patients and physicians alike (see Chapter 58). Although sclerotherapy and endovenous thermal ablation (EVA*) occupy preeminent roles in the contemporary management of superficial venous disease, surgical approaches remain relevant when applied appropriately and executed expertly.
In addition to treatment of the GSV and tributary varicosities, this chapter emphasizes management of the small saphenous vein (SSV) and other veins of the popliteal fossa; these veins are increasingly being recognized as important contributors to chronic venous insufficiency (CVI), and they are particularly well suited for treatment with open surgical techniques. In their recent publication, Gloviczki et al8 recommended specific diagnostic and treatment steps with the strength of recommendation and quality of evidence supporting that recommendation. Guideline recommendations specific to the open treatment of axial reflux and tributaries will be incorporated in the boxes in this chapter. The specific recommendations of this document will be designated by Varicose Vein Guide Lines (VVGL). These guidelines are based on Guyatt and associates’ Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, in which the strength of the recommendation is either strong (1) or weak (2). A grade of 1 means that we recommend (i.e., the benefits clearly outweigh the risks). A grade of 2 means that we suggest (i.e., the benefits are closely balanced with the risks). In this system, the strength or quality of evidence receives a letter grade A (highest), B, or C (lowest).
Decision Making
Natural History
Varicose veins constitute a progressive disease; remission of the disease does not occur, except after pregnancy and delivery. During its course the disease produces complications that usually prompt the patient to seek medical care. The most frequent complications are superficial thrombophlebitis; acute bleeding originating in one of the thin-walled varices; eczema; and finally, skin ulceration. Depending on age, general health condition, and symptomatology, patients with varicose veins may be offered one or more of the following: no treatment, conservative management with compression, sclerotherapy, or surgical treatment (open or endovascular). As noted in Chapters 55 and 56, CVI is extremely common in Western societies and affects approximately a third of the adult population. Fortunately, only a relatively small proportion of these patients develop the most severe form of venous insufficiency—namely venous ulcers. Though affecting only a small fraction of the population with venous insufficiency, venous ulcers are reported to occur in up to 4% of people older than 65 years.9,10 Even though nonoperative management of venous disease, including compression, elevation, and skin care, is clearly beneficial,11 such therapy does not correct the underlying pathology, allows immediate recurrence of symptoms if patients are unable to comply with the elevation and compression regimen, and is associated with a high incidence of recurrent ulceration and progression of disease.
Treatment Options
Interventional treatment of superficial venous incompetence can be accomplished by techniques that result in removal, ablation, or ligation of the refluxing venous segment. At the current time, reconstructive procedures have no role in the treatment of superficial venous disease outside of experimental protocols. Current options for eliminating this target reflux include high ligation, ligation and stripping (L&S), EVA, sclerotherapy, and ambulatory phlebectomy. Each of these techniques has a role in the treatment of patients with symptomatic varicose veins or CVI. The modern vascular surgeon should be comfortable in applying these procedures in a thoughtful manner based on the patient’s individual needs. As requested by insurers, a trial of compression treatment is generally employed with compression hose (20-30 mm Hg) for patients in CEAP classification C2 to C4 to determine whether the patient’s symptoms are relieved. For patients with an ulcer (CEAP C6) compression therapy with garments that exert 30-40 mm Hg is recommended, because the Effect of Surgery and Compression on Healing and Recurrence (ESCHAR) study showed that compression was comparable to ligation and stripping of the GSV in promoting healing.12
Patient Risk Assessment
When considering any intervention, the patient’s general medical status and venous anatomy should be considered. Because venous insufficiency results in chronic discomfort and stasis changes that may be ameliorated by the judicious use of compression garments, skin care, and lifestyle changes, venous surgery must always be considered somewhat elective, and accordingly, prudent consideration of general medical factors is indicated. In addition, the primary benefit of venous interventions is believed to be long-term improvement in symptom complex and ulcer recurrence. Patients who are the most fit, with longer life expectancies, have not only a reduced risk for complications from the procedure but also a longer period during which the benefits of the procedure may accrue. The advent of less invasive treatment by EVA has expanded the range of ages being treated; indeed, patients between 70 and 80 years of age are now undergoing therapy, usually for more severe and recalcitrant symptoms. Therefore patients in generally poor health with limited life expectancy or other severe comorbid conditions are typically best managed with conservative measures alone. In recognition of the shorter recovery time and reduced pain and morbidity, the VVGL recommends endovenous thermal ablation over open treatment of saphenous incompetence.

Anatomic Considerations
The lower extremity venous system is unique in its constant need to overcome the force of gravity while returning blood toward the heart. An elegant calf pump and vein valve system normally accomplish this task efficiently. The thin bicuspid, semilunar vein valves float open in the venous stream during prograde flow. During calf muscle relaxation, retrograde flow and higher supravalvular pressure cause the margins of the valve cusps to move to the midline and oppose so that caudad reflux is prevented. These valves can be disrupted by a thrombotic event (secondary), can be congenitally absent or atretic, or can become dysfunctional over time as the vein wall or valve loses integrity (primary). CVI has traditionally been classified on the basis of anatomy, function, and clinical severity. The anatomic classification of CVI is important because it links the location of CVI with its subsequent clinical management.
Great Saphenous Vein
The GSV arises anterior to the medial malleolus and courses obliquely and posteriorly as it crosses the anteromedial surface of the calf. At or below the knee joint, the posterior arch vein joins it. In the calf a solitary vein is found in about two thirds of cases, whereas a duplicated system is present in the remainder. In the vast majority of cases, the GSV at the calf level is anterior dominant. As the main saphenous trunk continues in a slightly more superficial plane around the knee joint, an anterior accessory vein often merges. The GSV then courses cephalad on top of the deep fascia and deep to the superficial fascia (Fig. 57-1). The GSV may run within this envelope for the entire length of the thigh or may enter at some distance above the knee. If the GSV is “duplicated,” both veins will run in this fascial envelope; there may be anterior and posterior accessory veins that enter the fascial envelope to join the GSV, but they exist primarily in the extrafascial plane. Three anatomic variants have been characterized and are demonstrated in Figure 57-2. Kupinski and coauthors reported their experience with duplex ultrasound (DUS) evaluation of the GSV in nearly 1500 limbs.13 This evaluation was divided between approximately 1200 GSV and 470 SSV examinations that were carried out in preparation for infrainguinal bypass. At the thigh level in 60% of the limbs the GSV had a single medial dominant system, whereas a branching double system was observed in nearly 20%, a complete double system in 10%, and a closed-loop system in another 10%. Of interest was a lateral dominant system, present in 8%.
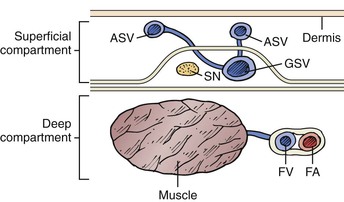
Figure 57-1 The saphenous compartment is bounded by deep and superficial layers of fascia. Tributaries to the saphenous vein pierce the superficial fascia, and it is they that become varicose. ASV, Accessory saphenous vein; FA, femoral artery; FV, femoral vein; GSV, great saphenous vein; SN, saphenous nerve.
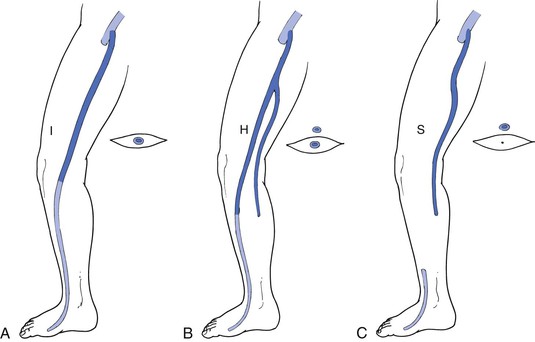
Figure 57-2 Anatomic types of the great saphenous vein (GSV) with respect to the fascial envelope. A, “I” type. The GSV is present within the fascial envelope along its entire length. B, “H” type. There is a subcutaneous collateral running parallel and superficial to the main saphenous trunk (left). C, “S” type. The caudal portion of the GSV in the thigh is atretic, and the extrafascial tributary is dominant.
Small Saphenous Vein
The confluence of the SSV with the popliteal vein has long been recognized as being variable (Fig. 57-3). Approximately 33% of SSVs terminate at a (high) above-knee popliteal vein site, whereas a low termination site is unusual (<10%).14–16 We have observed that DUS examination of the SSV allows exact perioperative and intraoperative localization of the saphenopopliteal junction for appropriate placement of the skin incision.
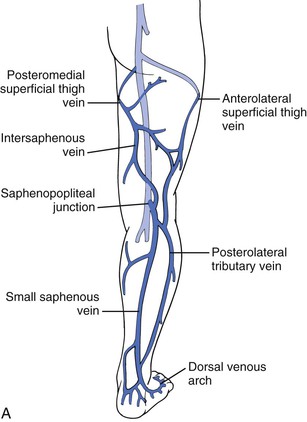
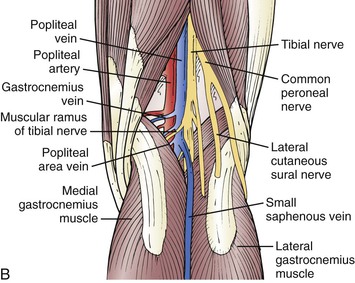
Figure 57-3 A, The small saphenous vein dominates the posterolateral superficial venous drainage and originates in the dorsal venous arch. At the posterolateral aspect of the ankle, it is intimately associated with the sural nerve. Note the important posterolateral tributary vein and the posterior thigh vein, which ascend and connect the small saphenous venous system with the GSV. The anterolateral superficial thigh vein and the posterolateral tributary vein can be very important in congenital venous anomalies, such as Klippel-Trenaunay syndrome. B, The most common neurovascular configuration of the popliteal fossa, although the site of anastomosis is highly variable.
Gastrocnemius Veins
The gastrocnemius veins arise from both the medial and lateral heads of the gastrocnemius muscle and may join the popliteal vein directly or merge with the SSV to form a common trunk and then enter the popliteal vein.17 Conversely, the SSV may join with the gastrocnemius vein to enter the popliteal vein. Hobbs and Vandendriessche reported a 20% incidence of incompetence of the gastrocnemius veins17—a proportion similar to that described by Gillet and colleagues in 180 operations for SSV reflux.18 If the SSV is ligated distal to its junction with a common trunk and an incompetent gastrocnemius vein, persistent reflux is ensured.
Intersaphenous Vein
The second important non-SSV vein of the popliteal fossa, the intersaphenous (Giacomini) vein, has been classically described as coursing up the posterior medial aspect of the thigh.19 The intersaphenous vein usually arises off the SSV either as a branch or as a truncal continuation of the SSV, and it courses along a subfascial plane to join the GSV more frequently (64%) than the deep venous system (45%).19 Reflux is less frequent in the intersaphenous vein than in either saphenous vein. The proportion of limbs with intersaphenous vein incompetence, however, is greatly increased when incompetence of the SSV is found alone (odds ratio of 11.94) or combined with GSV incompetence (odds ratio of 11.7),19 and this vein may facilitate reflux between the GSV and SSV.20 In our series a dominant and incompetent intersaphenous vein that formed a common trunk with the SSV was observed in 8.5% of limbs undergoing surgery for SSV reflux. Delis and colleagues’ DUS study of 818 limbs also showed a low 3% incidence of popliteal area veins,21 the third non-SSV vein of the popliteal fossa. By contrast, Dodd’s classic study of 444 operations in the popliteal fossa showed popliteal area veins in 177 cases (40%), and in 60% they were branches of the SSV (50%) or gastrocnemius veins.22 They directly entered the popliteal vein in 37% of cases.
Neurovascular Relationships in the Popliteal Fossa.
Surgical exploration of the popliteal fossa can be associated with postoperative neurologic disability and, rarely, disastrous vascular complications. Two motor nerves, the tibial (medial popliteal nerve) and occasionally a low-lying sciatic nerve, can be juxtaposed near the SSV and its termination with the popliteal vein. The tendon-like appearance of both motor nerves may falsely encourage the surgeon to perform a less delicate dissection or to retract more vigorously in a deeper plane than would be carried out when a nerve is clearly identified. The sensory sural nerve courses superficial to the SSV or may intertwine with it, and this nerve can also be damaged by retraction or dissection.
Perforating Veins
Perforating veins connect the superficial venous system to the deep venous system and are discussed in detail in Chapter 59. Incompetent perforating veins are most commonly observed about 5 to 10 cm above the medial malleolus. In normal limbs, perforating veins permit unidirectional flow of blood from the superficial to the deep venous system through a set of one-way valves. Perforating veins are either direct, permitting the superficial venous system to communicate directly with the main deep veins, or indirect, such that they connect with the deep veins by way of a muscular vein. Direct perforating veins are relatively constant in anatomic location, whereas indirect perforators are irregularly distributed. There are six groups of perforating veins in the leg;—those of the foot, ankle, leg, knee, thigh, and gluteal regions. According to the revised nomenclature, perforating veins are further described by anatomic location, such as medial, lateral, posterior, paratibial, and other descriptors (Fig. 57-4).
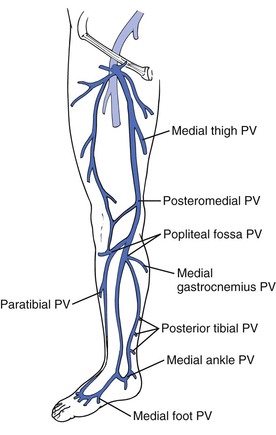
Figure 57-4 Important medial perforating veins (PVs) of the leg identified with the new anatomic nomenclature.
The medial leg perforating veins are clinically most significant. Cadaver studies have identified 7 to 20 medial calf perforating veins, with slightly more than half being direct perforators.23 These perforators connect the posterior accessory saphenous vein or other tributaries of the saphenous vein directly with the posterior tibial vein. Less than half of these perforators make direct connections from the GSV to the posterior tibial veins, with the majority connecting directly. In the thigh there are fewer perforating veins; however, they can be clinically very important. The medial thigh and femoral canal perforating veins communicate between the femoral vein or popliteal vein and the GSV either directly or indirectly.
Proximal Veins
The iliac veins are the primary venous outflow for the lower extremities. Venous valves are present in the iliac veins in approximately 27% of cases and are nearly twice as common on the right as on the left. When present, these valves can block reflux associated with a Valsalva maneuver and limit the utility of this maneuver in the diagnosis of lower extremity reflux. Compression of the left iliac vein as it crosses under the right iliac artery to reach the vena cava is termed May-Thurner syndrome. Iliac vein compression may result in increased resistance to flow and increased venous pressure. Finally, the inferior vena cava, which is the common outflow tract for both legs, is typically right sided, but congenital anomalies, including duplication and transposition, occur in approximately 1% of cases. Because the inferior vena cava does not contain valves, it is not implicated in reflux disease but is relevant in venous obstruction when recanalization or bypass is being contemplated.
Surgical Techniques
Surgery for superficial varicose veins should be individualized according to the patient’s preoperative evaluation. A combination of ligation, axial stripping, and stab phlebectomy may be applied as needed to the GSV, SSV, tributary veins, and perforating veins. Technical considerations for each of these techniques are summarized in the following sections.
After preoperative evaluation and elucidation of the necessary scope of surgery, a decision will be made regarding the appropriate method of anesthesia and site of service. Any of the surgical procedures can be performed under general or regional anesthesia in an operating theater, and this level of care may be appropriate for patients undergoing multiple incisions requiring extended procedure times or those with medical issues requiring close monitoring by an anesthesiologist. However, extensive experience in the use of tumescent local anesthetic techniques, with or without sedation, allows essentially any of these vein operations to be performed in an office procedure room if appropriately equipped and staffed. Ultimately, the decision is influenced primarily by local resources, physician experience, and patient expectations. Preoperative marking of the patient in the standing position with an indelible marker is important in any case in which stab phlebectomy or direct perforator ligation is contemplated. Such marking is essential because visualization of varicose tributaries may be impossible once the patient is prepared and the leg elevated. Patients are to be shaved immediately preoperatively with a clipper and the leg cleansed with an appropriate surgical preparation.
High Ligation of the Great Saphenous Vein
The GSV is most easily approached through an oblique incision 1 cm above and parallel to the groin crease. This location provides the best cosmetic results and the most reliable access to the saphenofemoral junction. The incision should start over the palpable femoral artery and extend medially to balance the better cosmesis of limited incisions with the necessity to ensure appropriate visualization of the saphenofemoral junction and its tributaries. Preoperative DUS-guided marking of the saphenofemoral junction further improves the precision of incision placement and allows minimal incision size and subcutaneous dissection. As the subcutaneous tissue is split, the main trunk of the GSV is identified. A self-retaining retractor is helpful, and the plane over the saphenous vein is extended toward the saphenofemoral junction. This anterior plane is generally free of encumbrances and allows exposure to the junction. Care should be taken to ligate a small branch of the femoral artery, which frequently crosses anterior to the saphenofemoral junction and can cause troublesome bleeding. Each of the tributaries is divided and ligated because the saphenofemoral junction needs to be clearly identified. Failure to clearly define the saphenofemoral junction has resulted in disastrous injuries to the femoral vein or artery.24,25
There are six main tributaries joining the GSV near its termination (Fig. 57-5). However, the number and position of these tributaries vary greatly, and therefore it is necessary to dissect the femoral vein 2 cm above and below the confluence to be sure that no additional tributaries join the femoral vein directly. Lateral and medial accessory saphenous veins may enter the main trunk between 2 and 20 cm below the confluence. When stripping is planned, these distal tributaries are avulsed, but if ligation alone is planned, the dissection should be extended caudal for approximately 10 cm to ensure division of these hidden tributaries. High ligation of the GSV is performed close to the femoral vein. Double ligation is generally performed on a proximal stump with the second ligation being a suture ligature. Care should be taken to avoid narrowing the femoral vein in the process. Equally important is to avoid leaving a long stump with a risk for thrombus formation and potential embolism. Alternatively, the GSV may be divided close to its termination and the femoral side closed with a two-layered monofilament suture. If high ligation alone is to be performed, one should resect the segment of GSV exposed in the surgical field, generally 5 to 10 cm in length. The incision is closed in layers by approximating the subcutaneous tissue with absorbable suture and the skin with absorbable subcuticular sutures. The requirement for sufficient exposure of the saphenous vein and its tributaries should be tempered by new data suggesting that extensive dissection results in local humeral changes and upregulation of vascular growth factors that lead to neovascularization, which in turn is believed to be an important cause of recurrent varicose veins.26
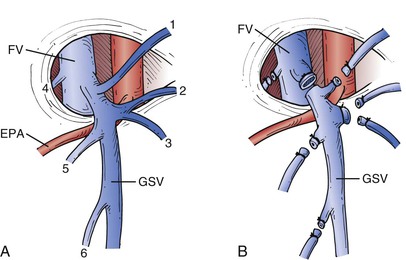
Figure 57-5 A, The most common arrangement of tributary veins at the saphenofemoral junction. The external pudendal artery (EPA) usually runs between the great saphenous vein (GSV) and the femoral vein (FV), but it may pass above the GSV and is then more susceptible to injury during dissection. 1, Inferior epigastric vein; 2, superficial circumflex iliac vein; 3, lateral accessory saphenous vein; 4, deep external pudendal vein; 5, superficial external pudendal vein; 6, medial accessory saphenous vein. B, All vessels must be individually divided and ligated to prevent recurrence. If no stripping of the GSV is to be performed, the proximal 5 to 10 cm of the GSV should be resected.
Great Saphenous Vein Stripping
GSV stripping is the central component of the classic operation for varicose veins. Recurrence rates are markedly reduced when the GSV is stripped as opposed to when high ligation is performed alone27,28; therefore high ligation is usually performed in conjunction with treatment of the GSV. During preoperative marking for L&S of the GSV, the surgeon should review the extent and distribution of reflux disease in the saphenous system. The GSV in the thigh is incompetent in only about two thirds of patients undergoing surgery for symptomatic varicose veins or CVI.29,30 In addition, unless the caudal below-knee saphenous vein is obviously incompetent and varicose, there is no need to remove it. Similarly, when H- or S-type anatomy is defined (see Fig. 57-2), it is not necessary to treat normal or atretic segments of the GSV, and in fact, such treatment may worsen the clinical situation by removing competent drainage paths (Fig. 57-6). This targeted approach to stripping leaves normal distal veins for potential future grafting, avoids injury to the saphenous nerve, and results in less postoperative pain and bruising without compromising the goals of surgery.31–33 If present, incompetent accessory saphenous veins should be addressed during the initial surgery. It is likely that the high recurrence rates noted for vein stripping in the past have resulted in part from unrecognized accessory veins. The availability of high-resolution DUS and awareness of the issue may result in improved efficacy in the future, and duplex definition of anatomy is essential in any modern-day operation on the great or small saphenous vein.
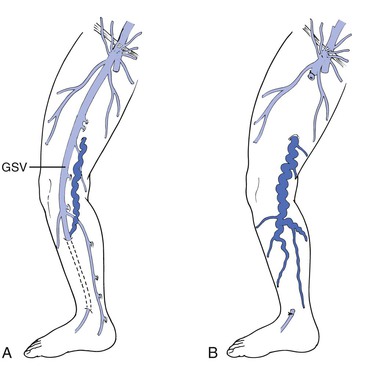
Figure 57-6 Stripping of a competent great saphenous vein (GSV) serving as runoff for a varicose collateral vein (A) results in postoperative worsening of the collateral varicosity because of resistance to outflow (B).
After flush ligation is performed, a transverse venotomy is created and a stripper is passed distally. Wire strippers or disposable plastic strippers are commonly used. In most cases the presence of reflux allows easy passage of the stripper to the level of the knee. A second small incision is made over the palpable stripper near the knee, which can be defined by preoperative or intraoperative duplex. The caudal incision is made transversely, and the subcutaneous tissues dissected to allow recovery of the saphenous vein. This top-down passage of the stripper not only facilitates identification of the saphenous vein at the knee and allows a small lower incision to be made but also avoids the potential for the stripper passed from below to enter the femoral vein through a thigh perforator and cause the femoral vein to be mistaken for the saphenous vein. The GSV should be stripped in a downward direction, which results in improved avulsion of tributaries and diminished injuries to the saphenous nerve.31 To avulse the vein with the endoluminal stripper, one needs to affix the catheter to the most cephalad portion of the vein. This may be accomplished by attaching the classic stripper head (Fig. 57-7) to the top of the disposable stripper after first placing a silk ligature around the vein and the stripper just below the head. Using the smallest head size minimizes tissue injury and bruising, whereas a larger head size will improve one’s chance of recovering the entire vein and the tributary segments. A long trailing silk suture is initially attached at the stripper head and drawn through the tunnel with the vein. After all the tributaries are avulsed and the caudal GSV divided and ligated, the vein is drawn back up to the groin incision, thereby minimizing the size of the distal incision.
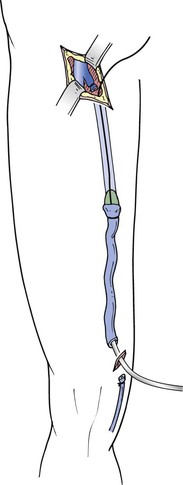
Figure 57-7 The intraluminal stripper is passed distally, the vein fixed by ligature proximally, and the stripping head secured. Stripping distally minimizes lymphatic and tributary damage and avoids avulsion of the saphenous nerve branch. When the stripper head reaches the distal incision, the distal vein fixed to the stripping device by ligature can be recovered through the proximal incision, thereby minimizing surgical trauma at the lower incision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


