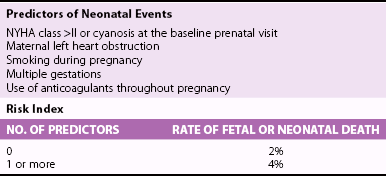
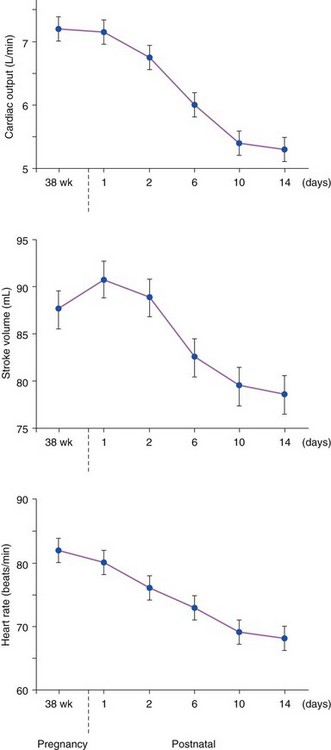
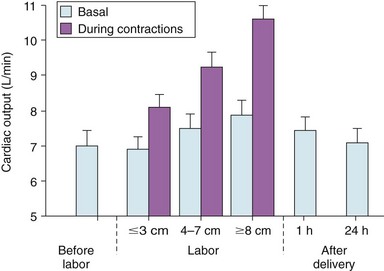
Chapter 27 During pregnancy, there is a substantial increase in plasma volume, erythrocyte volume, and cardiac output ( Figure 27-1).1–5 Cardiac output increases by up to 45%, and most of the increase is the result of a 20% to 30% increase in heart rate. There is a smaller increase in stroke volume.6–9 The increase in cardiac output begins as early as 10 weeks of gestation, with the maximal cardiac output achieved in most by 24 weeks ( Figure 27-2).8,10,11 Pulmonary pressures remain normal during pregnancy because of a decrease in pulmonary vascular resistance through vascular recruitment in the high capacitance pulmonary circulation. 12 Left ventricular (LV) filling pressures remain normal. 13 FIGURE 27-1 Plasma and erythrocyte increase during pregnancy. (From Pitkin PM. Clin Obstet Gynecol 1976;19:489–513, with permissions.) FIGURE 27-2 Increase in cardiac output from the nonpregnant state throughout pregnancy. During pregnancy, an increase in venous tone augments preload, 14 whereas a decrease in aortic stiffness and alterations in the microcirculation reduce afterload. 6 The decrease in systemic vascular resistance offsets the increase in cardiac output so that blood pressure decreases slightly during pregnancy. LV wall stress decreases by about 30%, which decreases the oxygen demand of the myocardium.4,15 Paradoxically, several studies suggest that LV contractility may be mildly depressed, although the magnitude of this change is unlikely to be clinically significant.4,15,16 Stroke volume is maintained in the setting of decreased contractility by the altered loading conditions of pregnancy. At term, the relationship between LV filling pressure and stroke-work index is comparable to the nonpregnant state. 17 The effects of positional change on hemodynamics also may be more prominent in women with valve disease. In the supine position, the gravid uterus can compress the inferior vena cava, resulting in decreased preload, stroke volume, and cardiac output. This can be avoided by use of the left lateral decubitus position. 18 Some patients may also need to labor in the left lateral decubitus position to maintain cardiac output. Peripartum hemodynamics are affected by uterine contractions, the pain of labor and delivery, and blood loss ( Figure 27-3). Pain increases heart rate, blood pressure, and stroke volume. Uterine contractions reintroduce blood into the circulating blood pool. The increase in intravascular volume with each contraction is accompanied by an increase in heart rate so that cardiac output is augmented by about 20% with each contraction ( Figure 27-4). When valve disease is present, these hemodynamic alterations may result in clinical deterioration.19,20 Labor and delivery are associated with mild increases in LV diastolic pressure, which, in a patient with decreased LV compliance, may lead to pulmonary edema. Therefore, the increase in intravascular volume that occurs with uterine contraction can increase LV end-diastolic pressure and pulmonary edema. FIGURE 27-3 Changes in heart rate and cardiac output after normal delivery. (From Hunter S, Robson SC. Br Heart J 1992;68:540–3, with permissions.) FIGURE 27-4 Changes in cardiac output and stroke volume during normal labor. (From Hunter S, Robson SC. Br Heart J 1992;68:540–3, with permissions.) Blood loss from vaginal delivery partially compensates for the increased blood volume of pregnancy, but acute changes may not be well tolerated in women with valvular heart disease. This is particularly true when the LV diastolic pressure-volume relationship is very steep, such as in women with severe aortic stenosis; a small loss of volume and preload may result in a large fall in cardiac output. However, the volume changes with a cesarean section are even greater than with vaginal delivery.5,21 Therefore, the cesarean section is rarely indicated for cardiac reasons. After delivery of the placenta, stroke volume and cardiac output rise by about 10% and remain elevated for about 24 hours. Over the next 2 weeks, cardiac output declines by 25% to 30% related to a decrease in heart rate and intravascular volume.22,23 In some patients, symptoms occur postpartum because of intravascular and extravascular volume shifts that result in spontaneous volume loading.17,24 Although hemodynamics return toward the baseline by 6 to 12 weeks after delivery, the new postpartum baseline may be different than prepregnancy hemodynamics. For example, both LV and aortic dimensions may remain slightly larger than the baseline.7,23 Echocardiographic findings reflect the normal physiologic changes of pregnancy. LV end-diastolic diameter increases by 2 to 3 mm, with no change in end-systolic dimension, so both fractional shortening and ejection fraction are increased compared to the baseline.25–31 In addition, both aortic root and LV outflow tract diameters increase by 1 to 2 mm, and this often persists after pregnancy.32,33 The left atrial area increases by about 2 cm2, 28 in association with an increase in serum atrial natriuretic peptide levels.34,35 There is a small increase in mitral annulus diameter and a larger increase in tricuspid annulus diameter. 28 A small pericardial effusion is seen in 25% of healthy women during pregnancy. 35 The increased cardiac output of pregnancy leads to increased transvalvular flow velocities. Aortic and LV outflow velocities increase by about 0.3 m/s. The transmitral early ventricular filling (E) velocity increases by 0 to 0.1 m/s, with an increase in the late ventricular filling (A) velocity of 0.1 to 0.2 m/s.8,28 The greater increase in A velocity compared to E velocity results in a shift from the normal E/A ratio seen in young adults to an equalized or reversed E/A ratio. The pulmonary venous flow pattern shows an increase in the velocity, but not duration, of the pulmonary venous A wave. 6 For these reasons, pregnant women can appear to have diastolic dysfunction on echocardiography. Mild tricuspid and pulmonic regurgitation are usually seen during pregnancy. Physiologic mitral regurgitation is also common, likely a result of annular dilation. 36 The incidence of rheumatic heart disease has declined in industrialized nations over the last 40 years.37,38 During that same time, there has been an increase in the number of adults with congenital heart disease. Additionally, other causes of valve disease, including connective tissue disorders such as Marfan syndrome, are more frequently recognized in pregnancy. Consequently, although rheumatic heart disease remains common in pregnancy in developing countries, in industrialized nations congenital and genetic valvulopathies are more common. This adds further complexity because patients with congenital or genetic valve disease frequently have associated cardiovascular abnormalities beyond the valve disease itself; some patients have a systemic right ventricle or aortic pathology that introduces additive risk. The spectrum of valve disease in pregnancy makes risk assessment somewhat difficult, but there are general risk factors that are identified, as well as risks based upon the specific valvular lesion. The European Society of Cardiology has created the Registry of Pregnancy and Cardiac disease and prospectively enrolled more than 1300 pregnant women with heart disease. In those with valvular heart disease, maternal mortality was 2.1% and hospitalization rate was 38%. 43 However, risk is not increased uniformly in all pregnancy women with valve disease. Accurately identifying risk factors for adverse outcomes is needed for prepregnancy counseling and decisions about appropriate monitoring during pregnancy. A multicenter Canadian study prospectively enrolled consecutive pregnancies in women with all types of heart disease. 39 Predictors of adverse maternal events were a history of cardiac events prior to pregnancy, New York Heart Association (NYHA) functional class greater than II, cyanosis, and left heart obstruction or systemic ventricular dysfunction. These four predictors allow prediction of the risk of maternal events ( Table 27-1 and Figure 27-5). In this series, the live birth rate was 98%. Maternal risk factors for fetal or neonatal death are shown in Table 27-2. Adverse neonatal events occurred in 20% of pregnancies, including premature birth in 18% and small-for-gestational-age birth weight in 4% of pregnancies. In women with congenital heart disease, but not a recognized genetic syndrome, 7% of infants had congenital heart disease. TABLE 27-1 Predictors of Primary Adverse Events * in Pregnant Women with Cardiac Disease 39 *Primary adverse events were defined as pulmonary edema, sustained symptomatic arrhythmia requiring treatment, stroke, cardiac arrest, or cardiac death. FIGURE 27-5 Frequency of maternal primary cardiac events. In this same study population, 302 pregnancies in women with heart disease were compared to 575 pregnancies in women without heart disease. The rate of maternal cardiac complications was 17% in women with heart disease, compared to 0% in the control group. Heart failure and arrhythmias accounted for most of the cardiac complications (94%). There were two postpartum maternal deaths as a result of heart failure or pulmonary hypertension. In addition, the risk of neonatal complications was 2.3 times normal ( Figure 27-6). The additive effects of maternal cardiac and obstetric risk factors support referral of these patients to high-risk obstetric clinics ( Figure 27-7). 40 FIGURE 27-6 Neonatal complications in women with and without heart disease. FIGURE 27-7 Effect of maternal risk factors on neonatal complication rates. There is an increasing number of women with complex congenital heart disease, which often includes valve dysfunction. In a report of 90 pregnancies in 54 women with various types of congenital heart disease, 41 risk factors for maternal events were NYHA class greater or equal to II, prior history of heart failure, and smoking. Severe pulmonic regurgitation (PR) or subpulmonic ventricular dysfunction also were risk factors for adverse maternal outcomes ( Table 27-3). Multivariate analysis identified LV outflow tract obstruction (peak outflow gradient greater than 30 mm Hg) as a risk factor for adverse fetal outcomes ( Table 27-4). Based on this data, a risk score for pregnant women with complex congenital heart disease was proposed ( Figure 27-8) that emphasizes the impact of PR and right ventricular dysfunction. TABLE 27-3 Comparison of Risks for Adverse Maternal Outcomes *The ACC/AHA guidelines are based on synthesis of data from multiple publications. TABLE 27-4 Comparison of Risk Factors for Fetal Complications NYHA, New York Heart Association. FIGURE 27-8 Risk score for adverse cardiac events during pregnancy. In another study of 1302 pregnancies in women with congenital heart disease, the overall rate of maternal complications was 7.6%. The strongest predictors of adverse maternal risk were presence of a mechanical valve, mitral or aortic stenosis, NYHA class greater than II, a history of arrhythmia, and the need for cardiac medications prior to pregnancy. Risk factors were additive and women with greater than 1 risk factors had a greater than 18% risk of a maternal complication during pregnancy (see Table 27-3). 42 Bicuspid aortic valve is associated with an aortopathy that shares many characteristics of Marfan syndrome (see Chapter 13) ( Figure 27-9). Congenital abnormalities, such as tetralogy of Fallot, transposition of the great vessels, and truncus arteriosus, may have associated aortic dilation. Some surgical repairs of valvular heart disease are associated with the development of aortic dilation, including the Ross repair of aortic stenosis or arterial switch repairs for transposition of the great vessels. The risk of dissection in congenital abnormalities does not appear to be as high as the risk in connective tissue disorders. Although the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines recommend consideration for aortic surgery to repair the aorta in patients with bicuspid valves and aortic diameter greater than 5.0 cm, 44 the aortic diameter that poses increased risk during pregnancy in such patients is not known. In those patients with Marfan syndrome, the risk appears highest for those patients with aortic diameters greater than 4.0 cm.45–47 FIGURE 27-9 Aortic dilation. Pregnancy increases the risk of dissection through several mechanisms, including estrogen interference with collagen deposition, elastase accelerating destruction of the elastic lamellae, and relaxin decreasing collagen synthesis.48,49 Aortic dissection during pregnancy is rare but appears to be more common in women with bicuspid aortic valve.48,50,51 These studies emphasize the importance of placing the valvular disease in the context of a patient’s other cardiac lesions, as risk factors for adverse outcomes may be additive. Additionally, functional class is important in risk assessment, independent of the underlying hemodynamic abnormality. Table 27-3 compares identified risk factors from the Valvular Heart Disease Guidelines of the ACC/AHA and the studies by Siu, Khairy, and Drenthen.39,41,42 Healthy pregnant women frequently have symptoms or exam findings that may be suggestive of heart disease ( Table 27-5). On exam, a systolic murmur is present in 80% of pregnant women and typically represents a benign flow murmur. 52 In 103 women without a previous cardiac history who were referred for echocardiography for a murmur appreciated during pregnancy, about 80% had a physical examination consistent with a flow murmur; all of these women had a normal echocardiogram. 52 In the 7% with a pansystolic, late systolic, or diastolic murmur, all had abnormal echocardiograms, including three ventricular septal defects, one large atrial septal defect, one atrial septal defect with rheumatic mitral regurgitation, and one nonobstructive hypertrophic cardiomyopathy. Echocardiography is usually adequate to characterize valve and ventricular function. However, in patients with poor acoustic windows or complex anatomy, transthoracic echocardiography may be inadequate. Transesophageal echocardiography may be a useful surrogate, depending on the information needed. Cardiac magnetic resonance imaging appears to be safe in pregnant women, particularly after the first trimester, and can accurately quantify valvular regurgitation, ventricular dimensions, and ventricular function. 53 Gadolinium contrast is not required for the evaluation of ventricular and valvular function. The safety of gadolinium has not been well documented in pregnancy, and, because it crosses the placenta, it is usually avoided in pregnancy. Occasionally, nonpharmacologic measures, such as bedrest, oxygen supplementation, avoiding the supine position, and patient education, can be effective at reducing symptoms. Diuretics and beta-blockers have been used extensively in pregnancy. Metoprolol undergoes accelerated metabolism during pregnancy so dose should be titrated to heart rate effect in conjunction with the obstetrics team. 54 Other beta-blockers have similarly accelerated metabolism, and many programs have found atenolol to be most effective, noting, however, that atenolol has a class D pregnancy rating from the U.S. Food and Drug Administration. Loop diuretics should be used to treat pulmonary congestion. However, diuretics can precipitate oligohydramnios so they should be used with caution. 55
Valvular Heart Disease in Pregnancy
Physiologic Changes of Pregnancy
Normal Hemodynamic Changes
Pregnancy
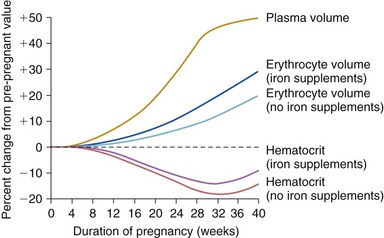
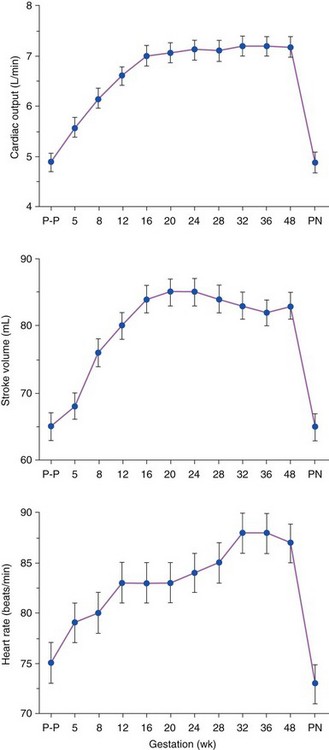
P-P, prepregnancy; PN, postnatal. (From Hunter S, Robson SC. Br Heart J 1992;68:540–3, with permissions.)
Positional Changes
Peripartum and Postpartum Changes
Evaluation by Echocardiography
Normal Anatomic Changes
Doppler Changes
Epidemiology
Risk Factors for Adverse Outcomes
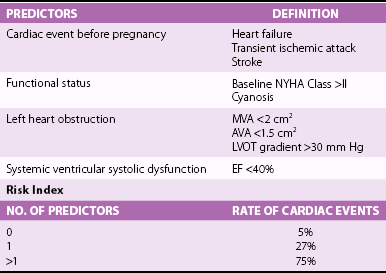
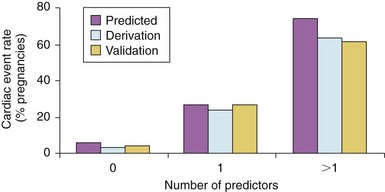
Frequencies are shown for derivation and validation groups, expressed as a function of the number of cardiac predictors, as shown in Table 27-1. (From Siu SC, Sermer M, Colman JM, et al. Circulation 2001;104:515–21, with permissions.)
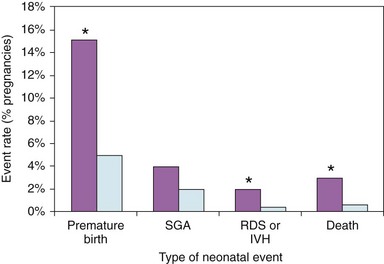
Specific types of neonatal complications in 302 pregnancies in women with heart disease (purple bars) are compared to 572 pregnancies in women without heart disease (light blue bars). Premature birth indicates delivery at <37 weeks of gestation. SGA, small for gestational age birth weight; RDS or IVH, respiratory distress syndrome or intraventricular hemorrhage; and death, fetal, or neonatal death. *P <0.005, heart disease versus controls. (From Siu SC, Colman JM, Sorensen S, et al. Circulation 2002;105:2179–84, with permissions.)
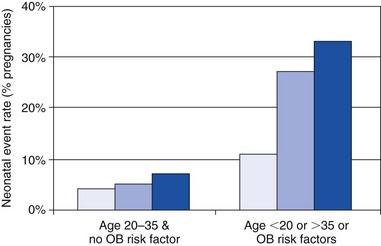
Frequency of neonatal complications when patients are divided into two groups by the presence or absence of maternal noncardiac risk factors (obstetric high-risk characteristics, including smoking, use of anticoagulation, multiple gestation, maternal age). Light blue bars represent control group. Medium blue bars represent the heart disease group without left heart obstruction or poor functional class/cyanosis. Dark blue bars represent the high-risk cardiac patients, with left heart obstruction or poor functional class/cyanosis. (From Siu SC, Colman JM, Sorensen S, et al. Circulation 2002;105:2179–84, with permissions.)
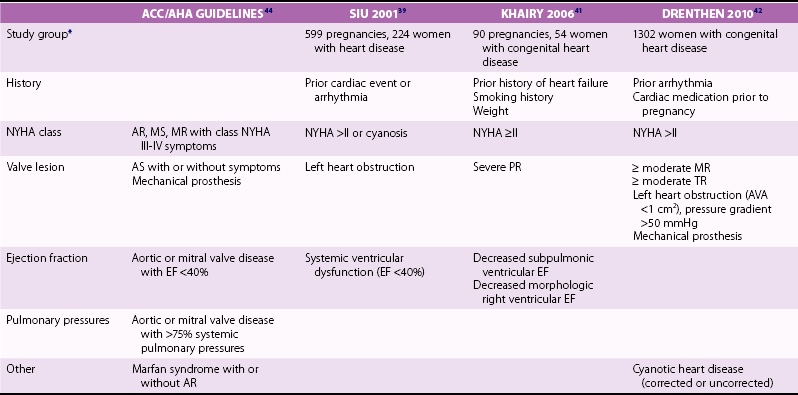
SIU 2001 39
KHAIRY 2006 41
DRENTHEN 2010 42
Cyanosis
Decreased saturation
Cyanosis
NYHA >II
Symptomatic arrhythmia
Cardiac medication prior to pregnancy
Left heart obstruction
Subaortic obstruction >30 mm Hg *
Smoking
Smoking
Smoking
Anticoagulation
Mechanical valve
Multiple gestation
Multiple gestation
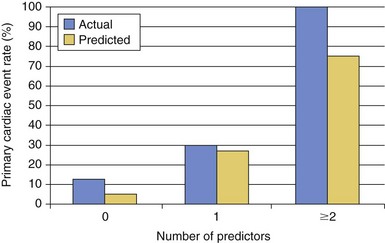
The actual versus the predicted event rates with 0, 1 and ≥2 risk factors. There was no significant difference between the actual and predicted groups. Risk factors in this study are shown in Table 27-4. (From Khairy P, Ouyang DW, Fernandes SM, et al. Circulation 2006;113:517–24, with permissions.)
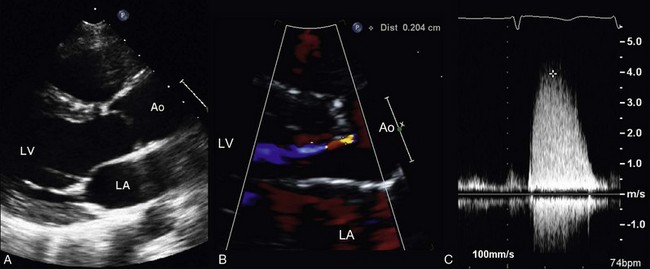
A 29-year-old woman who underwent a Ross repair for congenital aortic stenosis presents for preconceptual counseling. She is asymptomatic, able to keep up with her friends on long hikes and bike rides. Serial echocardiograms have shown progressive stenosis of her pulmonic homograft and enlargement of her proximal aorta (neo-aortic root). A, The parasternal long-axis 2D view shows a dilated aorta at the sinuses of Valsalva, with an end-diastolic diameter of 5.2 cm that has increased from 4.7 cm in the last 18 months. B, Color Doppler shows mild neo-aortic regurgitation. C, Continuous wave Doppler imaging of the pulmonic valve demonstrates severe pulmonic stenosis with a maximum velocity of 4.0 m/s. LV, left ventricle; LA, left atrium; Ao, Aorta.
Basic Clinical Approach
Evaluation of Disease Severity
Management During Pregnancy
Medical Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Valvular Heart Disease in Pregnancy
Only gold members can continue reading. Log In or Register to continue