Contrast-induced nephropathy (CIN) is a frequent, potentially lethal complication of percutaneous coronary interventions (PCIs). We prospectively validated the diagnostic performance of a simple CIN risk score in a large multicenter international cohort of patients who underwent PCI. About 2,882 consecutive patients treated with elective or urgent PCI were enrolled. A simple CIN risk score was calculated for all patients by allocating points according to a prespecified scale (pre-existing renal disease = 2; metformin use = 2; previous PCI = 1; peripheral arterial disease = 2; and injected volume of contrast medium ≥300 ml = 1). CIN was defined as an increase, compared with baseline, of serum creatinine by ≥25%, or by ≥0.5 mg/dl, 48 hours after PCI. CIN occurred in 15.7% of the study population. The predictive accuracy of the CIN risk score was good (c-statistic 0.741, 95% confidence interval 0.713 to 0.769). Receiver-operating characteristic analysis identified a score of ≥3 as having the best diagnostic accuracy. Examination of the performance of the proposed risk score using different definitions of CIN yielded a robust predictive ability. The score exhibited good discrimination (area under the curve ≥0.700) across all predefined subgroups of the study population. Compared with 2 previously published risk scores for CIN, our score demonstrated higher discriminative ability and resulted in a net reclassification improvement and an integrated discrimination improvement (p <0.001). In conclusion, the new risk score can easily be applied in the setting of urgent or elective PCI, allows for robust risk assessment and offers the potential to improve the peri-interventional management of patients at risk for CIN.
Contrast-induced nephropathy (CIN) is defined as an abrupt deterioration in renal function associated with the administration of iodinated contrast media. This type of acute kidney injury is frequently encountered as a complication of percutaneous coronary intervention (PCI) and is associated with adverse short- and long-term outcomes. Findings of prospective studies, in which patients with CIN had a 20-fold increase in mortality during their hospital stay, raise the possibility that CIN may independently contribute to an increased risk of early morbidity and mortality. Because there is no effective therapy once kidney injury has occurred, accurate and timely identification of patients at risk may have a substantial impact on the level of surveillance after PCI, the duration of hospitalization, and the implementation of prophylactic measures. Several clinical prediction rules and risk scores have been developed to predict the occurrence of CIN. However, their application in clinical practice is limited, either because they do not take into consideration the effects of recently proposed prophylactic interventions to prevent renal injury or because of their nongeneralizability. Recently, we proposed a simple risk score (the CIN risk score) to predict for CIN after PCI, accounting for co-morbidities, prophylactic measures, and preintervention treatment. In the present study, we prospectively validated the diagnostic performance of our CIN risk score model in a large multicenter international cohort of patients who underwent PCI.
Methods
We designed a multicenter, international, prospective cohort (noninterventional) study involving the cardiac catheterization laboratories of 6 tertiary centers across 4 countries (Greece = 2; Turkey = 2; Serbia = 1; and Spain = 1). Consecutive patients admitted for elective or urgent PCI between January 2010 and June 2012 were eligible for inclusion in the study. From the 2,882 patients screened, 26 were excluded because of long-term hemodialysis treatment. Patients who died within 48 hours of admission (n = 23), those referred for coronary artery bypass graft surgery during the index hospitalization (n = 51), those with missing creatinine values at 48 hours (n = 53), and those who needed repeated revascularization (n = 40) within 1 week of the index procedure were also not included in the analysis. The remaining 2,689 patients comprised the validation cohort. The Medical Ethics Committee of each 1 of the participating institutions approved the research protocol, and all included patients gave written informed consent.
Patients underwent PCI according to the current guidelines. The decision to implement renal prophylactic measures as well as the type of these measures ( N -acetylcysteine, hydration, sodium bicarbonate) was left to the discretion of the physicians caring for the patient. Platelet glycoprotein IIb/IIIa receptor inhibitors and intravenous inotropic support were administered, and intra-aortic balloon pump counterpulsation was performed, according to each institution’s clinical protocols. In accordance with the current guidelines, metformin was withheld for 48 hours before the procedure (for elective patients) and for 48 hours after PCI (for all patients). Nonionic or ionic, iso- or low-osmolality contrast agents were used during the revascularization procedures.
Serum creatinine concentrations were routinely measured at the time of admission (before PCI) and 48 hours after the procedure. The attending physicians completed a standardized questionnaire that contained demographic parameters, a detailed history of cardiovascular disease and other co-morbidities, procedural details (including the number of diseased coronary vessels, number of treated vessels, dose of contrast medium used, and duration of the procedure), antithrombotic and other treatment, and prophylactic measures to prevent the development of CIN for each patient.
The proposed risk scoring system (CIN risk score) consists of 5 variables ( Figure 1 ). All questions are categorically answered with a “yes” or a “no.” If a question is answered “yes,” points are allocated according to a prespecified scale (pre-existing renal disease = 2; metformin use = 2; previous PCI = 1; peripheral arterial disease = 2; contrast volume ≥300 ml = 1). The points for individual questions are then added to obtain the total score (range 0 to 8). If the total score is ≥3, the patient is considered to be at high risk for developing CIN.
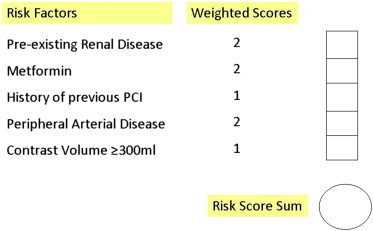
The proposed risk score has been derived from a cohort of 488 patients, 50 of whom (10.2%) developed CIN after coronary interventions. Briefly, the 5 predictive parameters of the risk score were selected of 57 demographic, clinical, angiographic, and procedural variables. The association between CIN and study variables was first evaluated using univariable logistic regression; significant predictors were selected for the final model in which multivariable logistic regression analysis with backward deletion method was performed to identify independent predictors of CIN. Points were allocated to each parameter based on the magnitude of the corresponding coefficients.
Definitions of various clinical terms used throughout the present manuscript are explained in Appendix Table 1 . CIN was defined as an increase (compared with the baseline values) of serum creatinine levels by at least 25% or at least 0.5 mg/dl 48 hours after PCI. Exploratory analysis of the prognostic power of the score was also performed using alternative definitions of CIN ( Appendix Table 2 ). Creatinine clearance was estimated using the Cockcroft-Gault method. Renal function was categorized according to the stages proposed by the National Kidney Foundation. Major bleeding was defined as proposed by the International Society on Thrombosis and Haemostasis.
Results for continuous variables are presented as means with SD and as percentages for categorical data. Normality was tested using the Shapiro-Wilk test. Differences in proportions were statistically evaluated using chi-square or Fisher exact test and trends in proportions using the Cochran-Armitage trend test. The association between the proposed risk score model and the occurrence of CIN was assessed by means of logistic regression analysis. For all logistic regression analysis models, odds ratios with 95% confidence intervals (CIs) were calculated. Model fit was assessed by the overall chi-square, the −2 Log likelihood, and the goodness of fit by Hosmer-Lemeshow statistic, whereas model predictive performance (discrimination) and calibration were assessed with the c-statistic and the calibration slope, respectively.
We determined the diagnostic accuracy of the proposed risk score by calculating the sensitivity, specificity, positive, and negative predictive values using the receiver-operating characteristic (ROC) curve analysis. Further, we assessed the diagnostic accuracy of the proposed risk score in predefined subgroups of clinical importance ( Appendix Table 4 ).We finally assessed the additive predictive value of the proposed risk score using reclassification tables, net reclassification improvement, and integrated discriminative improvement methods as described by Pencina. To elucidate the absolute and relative impact of the application of the new risk model on already established risk score models regarding the development of CIN, classification and regression tree analyses were conducted.
A p value <0.05 was considered to indicate statistical significance; all tests were two sided. The IBM PASW-SPSS Statistics 18.0 statistical software package (SPSS Inc., Chicago, Illinois) was used for all calculations with an exception of area under the curve (AUC) comparison for which MedCalc 19.2 Statistical Software (MedCalc Software, Mariakerke, Belgium) was used.
Results
Baseline demographic, clinical, and angiographic characteristics as well as main procedural data are listed in Table 1 . CIN occurred within 48 hours after procedure in 15.7% of the study population (423 of 2,689 patients). The proposed risk score for the development of CIN within 48 hours of PCI was characterized by a chi-square of 309, a −2 Log likelihood of 2,032, and a calibration slope of 0.96, p <0.001, whereas the associated risk per increase by 1 point exhibited n odds ratio of 1.74 with a corresponding 95% CI of 1.63 to 1.86 (p <0.001). The predictive accuracy of the CIN risk score was good as assessed by the c-statistic (AUC 0.741, 95% CI 0.713 to 0.769, p <0.001), with a Hosmer-Lemeshow statistic (degrees of freedom 3, chi-square = 4.8, p = 0.184) suggesting appropriate fit of the data. ROC analysis identified a score of ≥3 as having the best diagnostic accuracy in predicting the occurrence of CIN. This cut-off value was the same as the one previously identified within the derivation cohort. The overall sensitivity of the CIN risk score model was 67%, and the overall specificity was 76%. The CIN risk score model had a negative predictive value of 93% and a positive predictive value of 34%.
| Variable | Validation Study Cohort (n = 2,689) |
|---|---|
| Age (yrs) | 61 (12) |
| Age >70 yrs | 711 (29) |
| BMI (kg/m 2 ) | 28 (4) |
| Male/female | 1,885 (70)/804 (30) |
| Hypertension ∗ | 1,740 (65) |
| Diabetes mellitus | 789 (29) |
| Dyslipidemia † | 1,248 (46) |
| Current smoker | 1,203 (45) |
| Previous MI | 990 (37) |
| Pre-existing renal disease | 635 (24) |
| Baseline creatinine ≥1.5 mg/dl | 96 (4) |
| Chronic heart failure | 620 (23) |
| Ejection fraction <35% | 237 (9) |
| Peripheral arterial disease | 406 (15) |
| Previous stroke | 256 (10) |
| Atrial fibrillation | 230 (9) |
| Valvular disease | 482 (18) |
| Previous PCI | 983 (37) |
| Previous coronary bypass | 262 (10) |
| Anemia | 589 (22) |
| Baseline creatinine (mg/dl) | 1 (0.3) |
| Baseline hematocrit (%) | 41.1 (4.9) |
| Baseline hemoglobin (g/L) | 0.14 (0.02) |
| Baseline eGFR (ml/min) ‡ | 90 (31) |
| Baseline eGFR classification (ml/min) | |
| >90 | 1,279 (48) |
| 60–90 | 922 (34) |
| <60 | 488 (18) |
| Baseline systolic BP (mm Hg) | 128 (22) |
| Ejection fraction (%) | 51 (10) |
| Indication for the procedure | |
| Stable angina patients | 1,294 (48) |
| Acute coronary syndrome | 1,395 (52) |
| Type of procedure | |
| Elective procedure | 1,731 (64) |
| Urgent/emergency procedure | 958 (36) |
| Hydration infusion rate | |
| Only postprocedure hydration | 538 (20) |
| 0.5 ml/kg/h | 1,071 (40) |
| 1 ml/kg/h | 1,080 (40) |
| N -acetylcysteine use | 394 (15) |
| Sodium bicarbonate use | 119 (4) |
| No. of coronary arteries narrowed | |
| 1 | 1,030 (38) |
| 2 | 1,152 (43) |
| 3 | 502 (19) |
| PCI-treated vessels | |
| 1 | 2,472 (92) |
| 2 | 193 (7) |
| 3 | 14 (1) |
| Left main | 29 (1) |
| Bypass graft | 26 (1) |
| Duration of PCI (minutes) | 24 (23) |
| Number of stents used | 1 (0.7) |
| Contrast agent | |
| Volume contrast (ml) | 212 (109) |
| Volume contrast ≥300 ml | 502 (19) |
| Nonionic/ionic | 1,924 (72)/765 (28) |
| Low-/iso-osmolar | 2,638 (98)/51 (2) |
| Periprocedural complications | 152 (6) |
| Hemodynamic collapse § | 108 (4) |
| Use of inotropic support (catecholamine use or balloon counterpulsation) | 78 (3) |
| Major bleeding complications ¶ | 39 (2) |
| Blood transfusion(s) | 21 (1) |
| Vascular complications | 42 (2) |
| Previous medication use | |
| ACE inhibitor | 1,167 (43) |
| Angiotensin receptor blocker | 708 (26) |
| Renin inhibitor | 22 (1) |
| Diuretic | 598 (22) |
| Aldosterone antagonist | 77 (3) |
| Nitrate | 1,207 (45) |
| β blocker | 1,550 (58) |
| Calcium channel blocker | 530 (20) |
| Statin | 1,545 (58) |
| Fibrate | 172 (6) |
| Aspirin | 2,081 (77) |
| Clopidogrel | 1,405 (52) |
| Other antiplatelet agent | 156 (6) |
| Anticoagulant | 252 (9) |
| Metformin | 473 (18) |
| Other oral antidiabetics | 538 (20) |
| Insulin | 208 (8) |
∗ Defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or receiving antihypertensive treatment.
† Defined as low-density lipoprotein cholesterol >70 mg/dl or receiving hypolipidemic treatment.
‡ Calculated using the Cockcroft-Gault formula.
§ The PEITHO Steering Committee. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.
¶ Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in nonsurgical patients J Thromb Haemost 2005; 3:692–694.
The maximum observed score was 7 points; of note, the constructed model theoretically allows for a maximum score of up to 8. Approximately 85% of the patients had scores ≤3 ( Figure 2 ). The observed incidence of CIN for each score is shown in Figure 2 (Cochrane-Armitage test for trend with increasing score values; z score 18.2, p <0.001). The association between an increasing score and the predicted incidence of CIN suggests, as shown in Figure 3 , a quadratic rather than a linear effect.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


