Large thrombus burden (LTB) lesions in the context of primary percutaneous coronary intervention (p-PCI) have been related to unsuccessful angiographic reperfusion and unfavorable clinical outcomes. However, the hazard of LTB treatment on myocardial damage has not been evaluated. We investigated the impact of LTB on myocardial damage using contrast-enhanced cardiac magnetic resonance (CE-CMR) in the setting of p-PCI. In 327 patients, who underwent p-PCI without thrombus aspiration within 12 hours from symptom onset, we prospectively assessed the impact of LTB on infarct size and microvascular damage using CE-CMR. LTB was defined by the presence of Thrombolysis In Myocardial Infarction thrombus score ≥3 in patent infarct-related artery (IRA); or by “cut-off” occlusion pattern and/or large reference vessel diameter (≥3.5 mm) in occluded IRA. One hundred ninety-seven patients (60.2%) showed LTB and 130 (39.8%) did not. Distal embolization occurred in 18.8% patients with versus 6.9% without LTB (p = 0.003). At CE-CMR, patients with LTB had larger infarct size index (27.5 ± 11.1 vs 22.1 ± 17.5, p = 0.009) and more often transmural necrosis (70.5% vs 55.4%, p = 0.008) compared with patients without LTB. Excluding patients with distal embolization, patients with LTB still had larger necrosis. At multivariate analysis, occluded (IRA) at baseline, anterior infarction, and presence of LTB predicted transmural necrosis. In conclusion, LTB in the setting of p-PCI is related to larger myocardial damage as detected by CE-CMR, regardless of angiographic detectable distal embolization.
Experimental and clinical studies have shown that distal embolization (DE) complicating primary percutaneous coronary intervention (p-PCI) may affect myocardial reperfusion, limiting myocardial salvage and worsening the prognosis of ST elevation myocardial infarction (STEMI). Recently, we were able to demonstrate that DE occurs more frequently in the presence of specific angiographic features of large thrombus. However, the predicting value of large thrombus burden (LTB) on DE is not yet defined. Moreover, the impact of LTB on myocardial necrosis and microvascular damage in the setting of STEMI has not been evaluated. Nowadays contrast-enhanced cardiac magnetic resonance (CE-CMR) represents the most appropriate technique to value the presence and extent of myocardial and microvascular damage in the setting of reperfused STEMI. Thus, the aim of the present study was to investigate the impact of LTB on myocardial and microvascular damage, using CE-CMR in the setting of reperfused STEMI.
Methods
We prospectively assessed data from consecutive subjects included in a prospective registry from January 2008 to December 2011, enrolling patients undergoing p-PCI and CE-CMR at Padova University. Inclusion criteria of the registry were continuous chest pain for at least 20 minutes and within 12 hours of onset of pain and (1) ST-segment elevation ≥1 mm (0.1 mV) in ≥2 contiguous leads on the 12-lead electrocardiogram; (2) persistent ST-segment depression in precordial leads V 1 to V 4 , with or without ST-segment elevation in inferior or lateral leads; or (3) new-onset left bundle branch block. Exclusion criteria were use of adjunctive mechanical devices (both thrombectomy catheters and distal protection devices), previous myocardial infarction, electric or hemodynamic instability during hospitalization, and contraindications to contrast-enhanced magnetic resonance imaging. p-PCI was performed with standard technique by radial (preferred) or femoral approach according to physician choice. The infarct-related artery (IRA) was the only target of the procedure and coronary bare-metal or drug-eluting stents were used at discretion of the operators. The procedure was considered successful if Thrombolysis In Myocardial Infarction (TIMI) 3 in the target vessel and a residual stenosis of <20% at target site were obtained. Before the procedure all patients received aspirin 250 to 500 mg intravenously, thereafter 100 to 325 mg/day; heparin (70 U/kg) was given to maintain the activated clotting time of >250 seconds. Clopidogrel was given as soon as possible after hospital admission at a bolus dose of 600 mg and then at a dose of 75 mg/day. Abciximab was given according to the judgment of the operator in catheterization laboratories. All patients gave written informed consent for the procedure. The study complies with the Declaration of Helsinki, and the ethics review board of our institution approved the study protocol. Two experienced operators blinded to clinical data performed the angiographic analysis offline; in case of disagreement a third independent opinion was obtained. Thrombolysis In Myocardial Infarction flow grade and myocardial blush grade were assessed as previously described. Intracoronary thrombus at baseline was angiographically identified and scored in 5 degrees according to TIMI thrombus score. The angiographic pattern of coronary occlusion was defined on baseline angiogram as follows: (1) “cut-off” pattern, when there was an abrupt occlusion of the epicardial vessel; (2) tapered occlusion, when there was a vessel tapering just before the occlusion; and (3) persistent dye pattern, when there was a dye staining just proximally and/or distally to the occlusion. Quantitative coronary analysis was performed at baseline and after procedure, using the Coronary Quantification Package (Philips Medical Systems, Best, the Netherlands), as previously described. DE was defined as a distal filling defect with an abrupt occlusion in distal IRA or in ≥1 peripheral coronary branches of IRA, distal to the percutaneous coronary intervention site. According to our previous experience, LTB was defined according to the presence of ≥1 of the following: (1) presence of cut-off pattern of IRA occlusion, (2) large diameter ≥3.5 mm of occluded IRA, and (3) TIMI thrombus score ≥3 in case of patent IRA ( Figure 1 ). Patients were examined by 1.5-T scanner (Harmony; Siemens, Erlangen, Germany) implemented with a cardiologic software (MRease SYNGO 2002B; Siemens). The CE-CMR examination was performed 5 to 8 days after revascularization. Electrocardiogram-gated breath-hold cine images were acquired using a segmented steady-state free-precession pulse sequence (TrueFISP) in multiple short-axis views every 8 mm by encompassing the left ventricle from base to apex; vertical and horizontal long-axis views were also acquired. Typical in-plane resolution is 1.6 × 1.9 mm 2 , with slice thickness 8 mm; time of echo (TE) 1.75 ms, time of repetition (TR) 3.5 ms, flip angle 60°, matrix 256 × 256, slice thickness 8 mm, and gap 2 mm. Rest first-pass myocardial perfusion was performed during administration of a gadolinium-based contrast agent (MultiHance, 0.2 mmol/kg; Bracco, Milano, Italy), using a single-shot saturation recovery gradient-echo pulse sequence. Three short-axis slices were obtained per heartbeat, every 10 mm, covering the infarct area as seen during cine imaging (90° prepulse, TR 2.5 ms, TE 1.3 ms, flip angle 20°, slice thickness 10 mm, matrix 128 × 256, and number of excitations 1). Late gadolinium enhancement images were acquired 10 to 15 minutes after contrast administration, using a 2-dimensional segmented inversion-recovery gradient-echo pulse sequence with slice position identical to the cine images, including long-axis views. Sequence parameters were the following: TR 450 ms, TE 1.31 ms, flip angle 15°, slice thickness 8 mm, and gap 2 mm. The inversion time was set to null the signal of viable myocardium (typical range 250 to 300 ms). Cine, first-pass perfusion, and late gadolinium enhancement images were acquired during the same imaging session and matched using slice position; finally, the images were analyzed using a 17-segment model. On all short-axis cine slices, the endocardial and epicardial borders were outlined manually on end-diastolic and end-systolic images. First-pass perfusion was evaluated qualitatively according to 17-segment model. Persistent microvascular damage was finally evaluated on late gadolinium enhancement images as the presence of hypoenhancement region with decreased signal intensity. Extent of necrosis was judged as transmural if late gadolinium enhancement extended to ≥75% of thickness of the myocardial wall in ≥2 segments. For analysis of transmural extent of infarction, the 2 most basal and most distal slices were excluded, because segmental evaluation at these levels is not considered to be reliable because of the left ventricular outflow tract and partial-volume effect, respectively. The infarct size index has been quantified by manually drawing short-axis slices and has been expressed as percentage of left ventricle mass. The presence of persistent microvascular damage has been included in the infarcted area. All CE-CMR measurements were performed independently by 2 observers, who were blinded to clinical and procedural data. Major adverse events occurring during the hospitalization, including death, nonfatal reinfarction and stroke, were collected. Diagnosis of nonfatal reinfarction was based on typical chest pain and/or new ST-segment changes with troponin I level re-elevation. Stroke was defined by development of new cognitive or neurologic deficit confirmed by computed tomography or magnetic resonance imaging. Hypertension was defined as history of repeated measurements exceeding 140/90 mm Hg in blood pressure as well as the presence of established antihypertensive drug therapy at time of hospital admission. Hypercholesterolemia was defined as low-density lipoprotein cholesterol levels >190 mg/dl and/or established statin therapy at time of hospital admission. All patients underwent 2-dimensional transthoracic echocardiographic examination before discharge. Patients were divided into 2 groups according to the presence or the absence of LTB. Categorical data are expressed as numbers and percentages and were compared using the chi-square or Fisher’s exact test, as appropriate. Continuous variables are expressed as mean ± SD and compared using the Student t test. The potential predictors of transmural necrosis were evaluated one variable at a time with logistic regression. This model included age, gender, diabetes mellitus, anterior myocardial infarction, ischemic time, multivessel disease, patent IRA at baseline, reference vessel diameter, stent diameter, presence of collaterals, final TIMI flow grade 3, abciximab use, and LTB. Variables that showed significant association with transmural necrosis on univariate analysis (p <0.10) were entered in a multivariate logistic regression model; p <0.05 was considered statistically significant. The results of the univariate and multivariate analyses are reported as p value, odds ratios, and relative 95% confidence intervals. A p value of <0.05 with the 2-tailed test was considered to be statistically significant. Statistical analysis was carried out by SPSS software package, version 19.0 (SPSS Inc., Chicago, Illinois).

Results
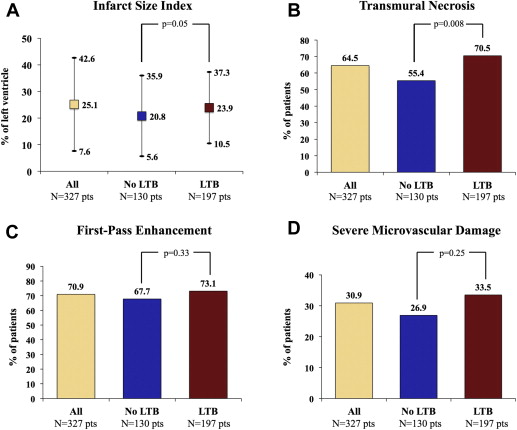
In the study period, 458 patients were treated by p-PCI at our institution; of these, 107 were excluded from the study because of thrombus aspiration, 27 because of contraindication to CE-CMR, and 70 because of both conditions. Thus, 327 patients were enrolled in the study, 197 patients (60.2%) had at least one or more features denoting LTB, and 130 (39.8%) did not. The rate of LTB in the group of 107 patients excluded from the study because of thrombus aspiration did not differ from that observed in the study population (55.9% vs 60.2%, respectively, p = 0.58). Clinical and angiographic characteristics are reported in Table 1 and Table 2 , respectively. Angiographic success was achieved in 90% of patients with LTB and 93% of patients without LTB (p = 0.23). Myocardial blush 2 or 3 grade was less frequently observed in patients with LTB ( Figure 2 ). At CE-CMR, patients with LTB showed larger infarct size index and trended toward more persistent microvascular damage ( Figure 3 ) compared with patients without LTB ( Figure 3 ). A subanalysis excluding patients with angiographically detectable DE was performed. The population was composed of 281 patients, 160 (56.9%) with LTB lesions and 121 (43.1%) without them. The analysis in this subset of patients showed results consistent with the overall population ( Figure 4 ). Correlation indexes (r) of transmural necrosis and/or microvascular damage and infarct size index measurements were, for interobserver variability, 0.94 and 0.95, respectively. Multivariate analysis identified anterior location of myocardial infarction, presence of LTB, and the patency of IRA at baseline as independent predictors of transmural necrosis ( Table 3 ). When multivariate analysis was restricted to patients with anterior infarction and occluded IRA, LTB still remained a predictor of transmural necrosis (odds ratio 2.27, 95% confidence interval 1.024 to 5.048, p = 0.043). In-hospital events were less and comparable between groups ( Table 4 ).
| Variable | Total, 327 (100%) | LTB | p Value | |
|---|---|---|---|---|
| Yes, 197 (60.2%) | No, 130 (39.8%) | |||
| Age (yrs) | 60.1 ± 11.9 | 60.1 ± 12.1 | 60.3 ± 11.7 | 0.87 |
| Men | 273 (83.5) | 166 (84.3) | 107 (82.3) | 0.64 |
| Diabetes mellitus | 58 (17.7) | 40 (20.3) | 18 (13.8) | 0.12 |
| Hypertension | 170 (52.0) | 105 (53.3) | 65 (50.0) | 0.59 |
| Hypercholesterolemia | 154 (47.1) | 99 (50.2) | 55 (42.3) | 0.19 |
| Smoker | 208 (63.6) | 120 (60.9) | 88 (67.7) | 0.20 |
| Anterior myocardial infarction | 174 (53.2) | 112 (56.8) | 62 (47.7) | 0.13 |
| Previous angina pectoris | 94 (28.7) | 50 (25.4) | 44 (33.8) | 0.20 |
| Previous heart failure | 2 (0.6) | 2 (1.0) | 0 (0) | 0.24 |
| Systolic blood pressure (mm Hg) | 137.1 ± 26.9 | 137.7 ± 28.0 | 136.2 ± 24.9 | 0.66 |
| Heart ratio (beats/min) | 75.1 ± 17.0 | 75.3 ± 17.6 | 74.8 ± 16.1 | 0.81 |
| Pain-to-balloon time (minutes) | 246.8 ± 183.6 | 235.7 ± 168.2 | 262.5 ± 204.8 | 0.21 |
| Troponin I peak (μg/L) | 117.3 ± 140.2 | 132.2 ± 153.7 | 93.5 ± 111.9 | 0.02 |
| Variable | Total (n = 327) | LTB | p Value | |
|---|---|---|---|---|
| Yes (n = 197) | No (n = 130) | |||
| Baseline angiographic data | ||||
| Multivessel coronary disease | 180 (55.0) | 105 (53.3) | 75 (57.7) | 0.41 |
| Infarct-related coronary artery | ||||
| Left anterior descending | 179 (54.7) | 119 (60.4) | 60 (46.2) | 0.04 |
| Left circumflex | 24 (7.3) | 14 (7.1) | 10 (7.6) | |
| Right | 124 (37.9) | 64 (32.5) | 60 (46.2) | |
| TIMI 2/3 before intervention | 109 (33.3) | 55 (27.9) | 54 (41.5) | 0.003 |
| Collateral circulation | 79 (24.1) | 46 (23.3) | 33 (23.5) | 0.69 |
| Quantitative angiography before PCI | ||||
| Diameter stenosis (%) | 92.6 ± 14.7 | 96.7 ± 7.9 | 86.4 ± 19.8 | <0.001 |
| Reference vessel diameter (mm) | 2.89 ± 1.06 | 3.00 ± 1.28 | 2.73 ± 0.56 | 0.03 |
| Minimal lumen diameter (mm) | 0.31 ± 0.75 | 0.22 ± 0.57 | 0.47 ± 0.93 | 0.003 |
| Lesion length (mm) | 18.4 ± 6.8 | 18.4 ± 7.5 | 18.5 ± 5.5 | 0.88 |
| Procedural data | ||||
| Stent diameter (mm) | 3.17 ± 0.47 | 3.23 ± 0.40 | 3.09 ± 0.55 | 0.09 |
| Total stent number | 1.25 ± 0.51 | 1.27 ± 0.51 | 1.20 ± 0.52 | 0.26 |
| Total stent length (mm) | 22.28 ± 10.6 | 22.80 ± 11.2 | 21.38 ± 9.7 | 0.24 |
| Stent/vessel | 1.04 ± 0.14 | 1.03 ± 0.11 | 1.06 ± 0.18 | 0.07 |
| Abciximab therapy | 125 (38.2) | 90 (45.7) | 35 (26.9) | 0.004 |
| Post-PCI angiographic data | ||||
| Final TIMI flow 3 | 298 (91.1) | 177 (89.8) | 121 (93.1) | 0.32 |
| Final myocardial blush 2/3 | 202 (61.8) | 109 (55.3) | 93 (71.5) | 0.007 |
| Distal embolization | 46 (14.1) | 37 (18.8) | 9 (6.9) | 0.003 |
| Angiographic no-reflow | 20 (6.1) | 14 (7.1) | 6 (4.6) | 0.36 |
| Quantitative angiography after PCI | ||||
| Diameter stenosis (%) | 10.9 ± 11.1 | 10.1 ± 6.5 | 12.0 ± 15.6 | 0.13 |
| Reference vessel diameter (mm) | 3.06 ± 0.52 | 3.18 ± 0.51 | 2.89 ± 0.47 | <0.001 |
| Minimal lumen diameter (mm) | 2.77 ± 0.56 | 2.89 ± 0.48 | 2.59 ± 0.62 | <0.001 |


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


