Our objective was to determine whether the Seattle Heart Failure Model (SHFM) differentiates patients with adult congenital heart disease (ACHD) at high versus low risk for cardiovascular outcomes and poor exercise capacity. The ACHD population is growing and presents increasingly for care in the community and at tertiary centers. Few strategies exist to identify the patients with ACHD at high risk for heart failure and mortality.We studied 153 adults with transposition of the great arteries, Ebstein anomaly, tetralogy of Fallot, double outlet right ventricle, and single ventricle from 2 ACHD centers. The primary outcome was cardiovascular death, with a secondary composite outcome of death, transplant, ventricular assist device, cardiovascular admission, and treatment for arrhythmia. We defined risk groups based on SHFM 5-year predicted survival: high (predicted survival <70%), intermediate (70% to 85%), and low risk (>85%). Ten patients had the primary outcome of death, and 46 the combined end point. The hazard of death in the SHFM high- versus the intermediate-risk group was 7.09 (95% confidence interval 1.5 to 33.4, p = 0.01; no deaths in the low-risk group) and the hazard of the composite outcome between the high- versus low-risk group was 6.64 (95% confidence interval 2.5 to 17.6, p = 0.0001). Kaplan-Meier survival analysis showed greater probability of all-cause mortality (p = 0.003) in the high-risk group. In conclusion, the SHFM can help identify subjects with ACHD at risk for adverse outcome and poor cardiopulmonary efficiency. This may add to the care of patients with ACHD in the community and streamline care at tertiary centers.
Patients with adult congenital heart disease (ACHD) are at increased risk for mortality and morbidity, with a significant incidence of heart failure. These patients are seen increasingly in local practices ; however, there is no comprehensive validated model to help cardiologists identify high-risk patients. Cardiopulmonary exercise testing (CPET) is used as a surrogate marker for outcomes in ACHD ; it, however, requires expensive equipment and specific expertise and is therefore not widely available to the general cardiologists caring for the ACHD population in the community. The Seattle Heart Failure Model (SHFM) is a validated prediction model that estimates survival in adult patients with heart failure by using commonly obtained clinical and laboratory variables. To determine whether the SHFM could serve as a useful prognostic model in the ACHD population, we evaluated the extent to which the SHFM differentiates high-risk from low-risk patients with ACHD and correlates with the CPET peak oxygen consumption (pVO 2 ) in a population of patients with ACHD at 2 tertiary referral centers.
Methods
We retrospectively assessed the medical records of 153 patients with ACHD (aged >18 years) with complex congenital heart diagnoses which carry a high risk of heart failure, including transposition of the great arteries with a systemic right ventricle (TGA; congenitally corrected TGA and atrial switch), Ebstein anomaly, tetralogy of Fallot (TOF), double outlet right ventricle, and single ventricle (SV) physiology, from 2008 to 2012 at the Massachusetts General Hospital (Boston, Massachusetts) and Montefiore Medical Center (Bronx, New York), who had sufficient documentation and data for analysis of SHFM and outcomes. All patients with TOF had their primary surgical repair before inclusion in the study. A subset of 112 patients had been referred by their cardiologist for CPET as part of routine clinical follow-up protocols. This study was approved by both institutions’ local ethics committees. All patients were monitored at least annually. The primary outcome of the study was cardiovascular death; the secondary outcome was a composite of cardiovascular death, listing for transplant, placement of a ventricular assist device, cardiovascular hospitalizations (for arrhythmia or congestive heart failure), and arrhythmia requiring treatment that occurred after the index clinic visit. These outcomes were also evaluated individually. Clinical and laboratory data obtained from medical records were used to calculate the SHFM for each patient. The SHFM includes the following variables: age, gender, New York Heart Association class, systemic ejection fraction, systolic blood pressure, ischemic versus nonischemic cause of cardiomyopathy, medications (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β blockers, hydroxymethylglutaryl–coenzyme A reductase inhibitors [statins], allopurinol, aldosterone inhibitors, diuretics [furosemide, bumetanide, metolazone, torsemide, and hydrochlorothiazide]), laboratory values (hemoglobin, percent lymphocytes, uric acid, sodium, and total cholesterol), and presence of a device (internal cardiac defibrillator, biventricular pacer, or biventricular pacer with internal cardiac defibrillator). It was derived from a population of 1,125 adults and validated in 9,942 patients with predominantly systolic heart failure. New York Heart Association class was based on clinical assessment documentation in the medical record nearest to the date of the CPET study within 1 year and confirmed with the primary cardiologist when clarification was necessary. Ejection fraction was obtained from review of echocardiographic data; magnetic resonance imaging data were used when available. When assessed by echocardiography, systemic ventricular ejection fraction was calculated by Simpson’s method. The SHFM score and predicted 5-year survival were calculated for each patient based on the published hazard ratios (HRs). Laboratory tests were obtained as clinically indicated. Patients with >2 missing variables were excluded. CPET with upright cycle ergometry and respiratory gas exchange was performed with previously reported methods. In brief, exercise testing was carried out after an overnight fast. Breath-to-breath respiratory gas exchange was continuously measured with a metabolic cart interfaced to the ergometer (Medical Graphics Corp., St. Paul, Minnesota used in the Massachusetts General Hospital laboratory, and Amis 2000, Innovision, Odense, Denmark used in the Montefiore laboratory). Peak VO 2 was defined as the highest oxygen uptake measured during the last minute of symptom-limited exercise. Percentage pVO 2 was calculated using population means for a given subject’s age, gender, and weight. Ventilatory efficiency (VE/VCO 2 ), the ratio between ventilation and carbon dioxide production, was calculated by linear regression of the minute ventilation and carbon dioxide production throughout the duration of exercise.
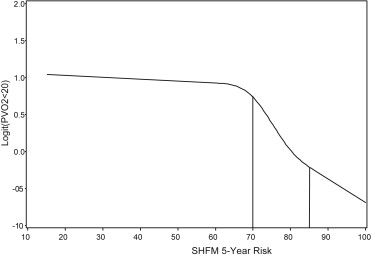
To facilitate the translation of SHFM survival values into clinically meaningful information in the ACHD population, we defined 3 risk groups: high risk (SHFM 5-year predicted survival <70%), intermediate risk (70% to 85%), and low risk (>85%). It is important to recognize that the SHFM survival percentages are not likely to represent actual ACHD survival, but serve as numerical cut points to assess high versus low risk of an event. Cut-off values for risk groups were created based on examining the functional relationship of SHFM 5-year predicted survival and pVO 2 on CPET using cubic splines ( Figure 1 ). Cox proportional hazards model was used to generate hazard ratios to compare groups by level of risk. Patients were stratified by SHFM-defined risk groups and compared for the occurrence of the primary and composite end points and individual components during follow-up by way of the log-rank test. In a Bayesian framework, interval likelihood ratios allow clinicians to estimate the probability of an event taking into account the results of a diagnostic test; in our case, the test is the SHFM. Interval likelihood ratios were calculated for the 3 groups for predicting pVO 2 <20 on CPET. Logistic regression was used to examine the association between the SHFM score with CPET characteristics that identify high-risk patients : pVO 2 <20 cc/kg/min, VE/VCO 2 slope >34, and % predicted pVO 2 <50% for the entire population, as well as separately for the distinct ACHD lesions. Model discrimination was estimated by the area under the receiver operator characteristic curve. Center-specific median values were imputed for subjects with <2 missing variables to replicate most closely the practical use of the SHFM. Two-sided p values <0.05 were considered statistically significant. Analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, North Carolina).
Results
Baseline characteristics can be found in Table 1 and Supplemental appendix Table 1 . One hundred fifty-three patients (TOF, double outlet right ventricle, Ebstein, TGA, and SV) were studied. Average follow-up time was 2.2 years (SD 1.4). Of the 112 individuals with CPET testing, the median pVO 2 was 19.9 cc/kg/min (interquartile range 15.4 to 26.1). Because CPET results were not available in the entire population, we performed an analysis to determine if patients with CPET data were systematically different from those without, as an assessment of potential selection bias. There were no significant differences in patient characteristics or outcomes for those with and without CPET data, although there could be a trend toward indicators of worse clinical status in patients who did not undergo CPET (data not shown). CPET results by diagnostic subgroups are reported in Table 2 . The mean SHFM score was 0.20 for all subjects with ACHD studied, with a higher score representing worse predicted survival. The mean SHFM score was 0.07 for TGA, 0.11 for TOF, 0.41 for Ebstein, and 0.59 for SV. Ten patients had the primary outcome of death, and 46 patients had the composite secondary outcome measure of death, transplant, ventricular assist device placement, cardiovascular admission, and treatment for arrhythmia. Twenty-eight patients had at least 1 cardiovascular hospitalization. Of the 10 deaths, 6 were related to advanced heart failure and all were due to a cardiovascular cause. One patient had implantation of a ventricular assist device; no patients received a transplant. Of the 26 patients with arrhythmias requiring treatment, most had supraventricular tachycardias; 2 patients were treated for ventricular tachycardia.
| Variable | All (n = 153) |
|---|---|
| Median age (yrs; range, SD) | 37.1 (18–69, 12.7) |
| Women | 53.6% |
| NYHA ≥2 | 54% |
| Median systemic ventricular EF (median ± SD) | 55% ± 11% |
| Mean hemoglobin (g/dl) | 14.0 |
| Mean sodium (mmol/L) | 139.1 |
| Pacemaker | 25 (16%) |
| Implantable defibrillator | 4 (3%) |
| Biventricular pacemaker-defibrillator | 2 (1.5%) |
| Medications | |
| ACE inhibitor or ARB | 32.7% |
| β Blockers | 41.2% |
| Statins | 14.4% |
| Diuretic | 22.9% |
| CPET Variables | All | TOF | TGA | Ebstein | SV |
|---|---|---|---|---|---|
| Median peak VO 2 (cc/kg/min; interquartile range) | 19.9 (15.4–26) | 20.0 (15.8–25.2) | 24.8 (17.7–27.6) | 19.0 (14.3–25) | 17.4 (14.8–22.4) |
| Median % predicted peak VO 2 (interquartile range) | 57 (49–69) | 57 (52–66) | 57 (48–71) | 59 (50–70) | 51 (38–57) |
| Mean VE/VCO 2 slope (interquartile range) | 30 (28–36) | 30 (26–33) | 33.1 (31–36) | 33.9 (31–37) | 39.1 (35–42) |
Patients in the SHFM low-risk group had significantly lower rates of the primary outcome in follow-up compared with those in the SHFM high-risk group (log-rank p ≤0.001, Figure 2 ). Similarly, they had significantly lower rates of death and cardiovascular hospitalizations but not of arrhythmia requiring treatment ( Figure 2 ). Conversely, patients in the SHFM high-risk group were significantly more likely to have the composite outcome compared with those in the SHFM low-risk group (HR 6.64, 95% confidence interval [CI] 2.50 to 17.62, p = 0.0001; Table 3 ). There were no deaths in the low-risk SHFM group. There was an increased risk of death in the high- versus intermediate-risk group (HR 7.09, 95% CI 1.50 to 33.41, p = 0.0133). For each 10% increase in the SHFM-predicted 5-year survival, the hazard of the composite outcome was reduced by 25% (HR 0.75, 95% CI 0.66 to 0.86, p <0.001) and that of death was reduced by 42% (HR 0.58, 95% CI 0.45 to 0.75, p <0.001). Currently, pVO 2 by CPET is one of the most commonly used prognostic markers of poor outcome in patients with heart failure. SHFM correlated well with pVO 2 on CPET in patients with ACHD: SHFM high-risk patients had a 2.3-fold increase in likelihood of having a pVO 2 <20 ( Table 4 ). By logistic regression, the odds of a pVO 2 <20 cc/kg/min doubled for each unit increase in SHFM score (odds ratio 2.06, 95% CI 1.19 to 3.59, p = 0.01). Similar associations with SHFM score were found for percent-predicted pVO 2 <50% and VE/VCO 2 (see Supplemental appendix Tables 2 and 3 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


