Inflammation plays a crucial role in the pathogenesis of in-stent restenosis (ISR). Neutrophil-to-lymphocyte ratio (NLR) provides a simple method for assessment of inflammatory status and prognosis in patients with coronary artery disease. The aim of the present study was to investigate the predictive value of preprocedural NLR on development of ISR in patients undergoing coronary stent implantation. We retrospectively analyzed clinical, hematologic, and angiographic data of 624 patients (mean age 60.5 ± 10.2 years, 71.8% men) who had undergone coronary stent implantation and a further control coronary angiography owing to stable or unstable angina pectoris. Patients were divided into 3 tertiles based on preprocedural NLR. Restenosis occurred in 21 patients (10.1%) in the lowest tertile, in 62 (29.8%) in the middle tertile, and in 107 (51.4%) in the highest NLR tertile (p <0.001). Serum C-reactive protein levels were also significantly higher in patients in tertile 3 than in those in tertiles 1 and 2 (p <0.001). Using multiple logistic regression analysis, smoking, diabetes mellitus, stent length, preprocedural NLR, and C-reactive protein levels emerged as independent predictors of ISR. In receiver operating characteristics curve analysis, NLR >2.73 had 80% sensitivity and 75% specificity in predicting ISR. In conclusion, high preprocedural NLR is a powerful and independent predictor of bare-metal stent restenosis in patients with stable and unstable angina pectoris.
Despite major advances in interventional techniques and drug therapies, in-stent restenosis (ISR) remains a major problem in interventional cardiology. The inflammatory process plays an important role not only in initiation and progression of atherosclerosis but also in development of stent restenosis. Because of inflammation, neointimal proliferation develops, which is the main cause of stent restenosis. White blood cell (WBC) subtypes, especially the neutrophil-to-lymphocyte ratio (NLR), has been proposed as a prognostic marker and seemed to be related to a proinflammatory state imposing worse clinical outcomes in cardiovascular disease. Various inflammatory biomarkers have been used in clinical practice to investigate the relation between inflammatory response and ISR ; however, no study has investigated any possible association between NLR and ISR. The aim of the present study was to evaluate the usefulness of NLR before successful bare-metal stent implantation in predicting ISR in patients with stable and unstable angina pectoris.
Methods
We analyzed clinical and angiographic data of patients who had undergone successful bare-metal stent implantation because of stable or unstable angina pectoris from February 2008 through June 2010 at Turkey Yuksek Ihtisas Educational and Research Hospital (Ankara, Turkey). For patients’ data, we gained access retrospectively into the data at the time of interest when the patients underwent coronary stent implantation after control coronary angiography performed because of anginal symptoms and positive treadmill test results, thus recalling clinical, angiographic, and laboratory characteristics at that time. As such, we were able to collect the data of 635 patients. Patients with unstable angina pectoris were identified according to the definition of Braunwald. As part of our preprocedural protocol, complete WBC and peripheral differentials had already been available before coronary angiography for all patients. Patients were excluded from analysis if they had clinical evidence of cancer (n = 2), chronic inflammatory disease (n = 4), or any active infectious disease (n = 5), leaving 624 patients to be included the study.
Patients’ clinical and demographic characteristics encompassing age, gender, history of arterial hypertension, diabetes mellitus, tobacco use, family history of coronary artery disease (CAD), left ventricular ejection fraction, and medications used were noted. In addition, serum levels of C-reactive protein (CRP), fasting blood glucose level, creatinine level, and fasting serum lipid status including total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride levels were also recorded. The local ethics committee approved the study protocol.
All laboratory data were obtained from venous blood samples up to 6 hours before stent implantation. Total WBC, neutrophil, lymphocyte, and monocyte counts were calculated using an automated blood cell counter (ADVIA 2120i Hematology System, Siemens Healthcare Diagnostics, Deerfield, Illinois). NLR was calculated as the preprocedural ratio of neutrophils to lymphocytes, which were obtained from the same blood samples. CRP levels were measured by an immunonephelometric method (Roche Diagnostics GmbH, Marburg, Germany).
Coronary interventions were performed according to current practice guidelines and recorded in digital storage for quantitative analysis. Degree of coronary stenosis was visually estimated by experienced interventional cardiologists. A luminal narrowing >50% in a major subepicardial vessel (left anterior descending, left circumflex, or right coronary artery) was defined as significant stenosis. Each patient received aspirin plus clopidogrel (loading dose 300 or 600 mg) before or during coronary intervention. Unfractionated heparin 100 U/kg was administered at the beginning of the procedure to keep the activated clotting time >200 seconds. Access site for percutaneous coronary intervention (PCI) was at the physician’s preference (femoral or radial). Usage of glycoprotein IIb/IIIa inhibitors and predilatation or postdilatation after stent implantation of the lesion was at the operator’s discretion. Successful PCI was defined as <20% decrease in diameter stenosis and residual stenosis <50% in diameter with final Thrombolysis In Myocardial Infarction grade 3 flow without any major complication. After stent placement, clopidogrel was used for ≥1 year and aspirin was used indefinitely. During routine clinical follow-up, coronary angiography was performed secondarily in patients with stable or unstable angina pectoris. Control coronary angiograms were recorded with Judkins technique and interpreted by 2 independent cardiologists who were blinded to patients’ data. Stent restenosis was accepted as narrowing >50% in an otherwise normal diameter, including 5 mm proximal and distal to the stent edge, according to results of control coronary angiographies. Intra- and interobserver variabilities of stent restenosis analysis were minimal in a representative subset of 70 patients. Interpretations of the 2 investigators on the presence or absence of ISR agreed in 94% (66 of 70) and 97% (68 of 70), respectively. Intraobserver variability was assessed by 1 investigator. The 2 readings were concordant for the presence or absence ISR in 96% (67 of 70) and 97% (68 of 70), respectively.
Analyses were performed using SPSS 13.0 (SPSS, Inc., Chicago, Illinois). Continuous data were presented as median and interquartile range or mean ± SD. To test the distribution pattern, the Kolmogorov–Smirnov test was used. The study population was assigned into tertiles based on NLRs at admission. Comparisons of multiple mean values were carried out by Kruskal–Wallis tests or analysis of variance as appropriate. Categorical variables were summarized as percentages and compared with chi-square test. Spearman correlation coefficient was computed to examine the association between 2 continuous variables. Effects of different variables on ISR were calculated in univariate analysis for each. Variables for which the unadjusted p value was <0.10 in logistic regression analysis were identified as potential risk markers and included in the full model. We reduced the model using stepwise multivariate logistic regression analyses and eliminated potential risk markers using likelihood ratio tests. A p value <0.05 was considered statistically significant and the confidence interval was 95%. Analysis was performed in 2 models. Preprocedural NLR was assumed to be a continuous variable in the first model and a categorical variable in the second model. An exploratory evaluation of additional cut points was performed using receiver operating characteristics curve analysis. A p value <0.05 was considered statistically significant.
Results
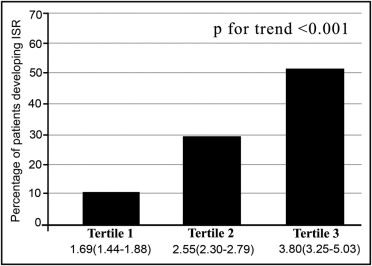
In total 624 patients with a mean age of 60.5 ± 10.2 years were enrolled in the study. Patients were divided into 3 tertiles based on NLR: 1.69 (1.44 to 1.88) in tertile 1, 2.55 (2.30 to 2.79) in tertile 2, and 3.80 (3.25 to 5.03) in tertile 3. Each group was composed of 208 patients. Table 1 presents baseline demographic and clinical data of patients by tertile of NLR.
| Variables | NLR | p Value | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| 1.69 (1.44–1.88) | 2.55 (2.30–2.79) | 3.80 (3.25–5.03) | ||
| (n = 208) | (n = 208) | (n = 208) | ||
| Age (years) | 59.2 ± 9.0 | 61.2 ± 10.2 | 60.7 ± 10.5 | 0.13 |
| Men | 141 (67%) | 145 (69%) | 160 (76%) | 0.09 |
| Family history of coronary artery disease | 84 (40%) | 87 (42%) | 96 (46%) | 0.465 |
| Diabetes mellitus | 59 (28%) | 64 (30%) | 73 (35%) | 0.017 |
| Current smoker | 77 (37%) | 90 (43%) | 116 (55%) | <0.001 |
| Hypertension | 112 (53%) | 123 (59%) | 129 (62%) | 0.230 |
| Cause of stent implantation | ||||
| Stable angina pectoris | 163 (79%) | 155 (75%) | 118 (57%) | <0.001 |
| Unstable angina pectoris | 45 (21%) | 53 (25%) | 90 (43%) | <0.001 |
| Number of coronary arteries narrowed | ||||
| 1 | 58 (27%) | 50 (24%) | 43 (21%) | 0.04 |
| >1 | 150 (73%) | 158 (76%) | 165 (79%) | 0.04 |
| Target coronary artery | ||||
| Left anterior descending | 104 (50%) | 91 (43%) | 104 (50%) | <0.001 |
| Right | 62 (29%) | 66 (31%) | 40 (19%) | <0.001 |
| Left circumflex | 42 (21%) | 51 (24%) | 64 (31%) | <0.001 |
| Stent diameter (mm) | 3 (2.75–3) | 3 (2.5–3) | 3 (2.5–3) | 0.551 |
| Stent length (mm) | 15 (14–16) | 15 (13–18) | 15 (12–20) | 0.321 |
| In-hospital medical treatment | ||||
| β Blocker | 79.3% | 80.9% | 76.1% | 0.63 |
| Angiotensin-converting enzyme inhibitor | 67.6% | 71.3% | 70.1% | 0.66 |
| Calcium channel blocker | 4.1% | 3.3% | 3.1% | 0.60 |
| Angiotensin receptor blocker | 5.9% | 6.8% | 6.0% | 0.52 |
| Statins | 86.4% | 89.4% | 87.5% | 0.68 |
| Left ventricular ejection fraction (%) | 58 (53–60) | 56 (53–60) | 55 (50–60) | 0.03 |
| Fasting glucose (mg/dl) | 89 (74–100) | 91 (71–102) | 94 (79–110) | 0.24 |
| High-density lipoprotein cholesterol (mg/dl) | 41 (35–50) | 40 (32–45) | 38 (32–44) | 0.001 |
| Low-density lipoprotein cholesterol (mg/dl) | 98 (75–130) | 104 (81–126) | 114 (90–138) | 0.001 |
| Triglycerides (mg/dl) | 134 (100–180) | 136 (97–190) | 149 (104–200) | 0.02 |
| Hemoglobin (g/L) | 14.25 (13.3–15.2) | 14.01 (13–15) | 13.9 (12.5–15) | 0.06 |
| Platelet count (×10 9 /L) | 244 (204–289) | 258 (219–316) | 259 (210–305) | 0.11 |
| Total white blood cell count (×10 9 /L) | 7.05 (6.2–8.9) | 8.5 (6.8–9.5) | 9.9 (7.9–10.8) | <0.001 |
| Neutrophil count (×10 9 /L) | 4.1 (3.2–5.2) | 5.2 (4.3–6.3) | 7.4 (5.7–8.3) | <0.001 |
| Lymphocyte count (×10 9 /L) | 2.45 (2.2–3.6) | 2.0 (1.8–2.4) | 1.6 (1.3–2.2) | <0.001 |
| Monocyte count (×10 9 /L) | 5.1 (4.3–6.1) | 6.2 (4.5–7.4) | 6.3 (5.2–7.3) | 0.002 |
| Neutrophils (percent white blood cells) | 56 (53.2–58.7) | 64.7 (62–66.8) | 72.6 (69.1–76.8) | <0.001 |
| Lymphocytes (percent white blood cells) | 33.2 (31.1–37) | 26.4 (25.8–27.1) | 18.9 (14.8–21) | <0.001 |
| Monocytes (percent white blood cells) | 6.95 (5.71–8.5) | 6.9 (5.7–8.1) | 6.3 (5–7.9) | 0.01 |
| C-reactive protein (mg/L) | 1.85 (0.72–3.55) | 2.8 (1.2–6.45) | 5 (3.35–18.2) | 0.001 |
| Period between 2 coronary angiographies (months) | 14.5 ± 2.4 | 13.9 ± 3.2 | 12.5 ± 3.1 | 0.009 |
| In-stent restenosis | 21 (10.1%) | 62 (29.8%) | 107 (51.4%) | <0.001 |
Patients in tertile 3 were more likely to have higher total WBC and differentials and higher CRP levels than those in tertiles 1 and 2. A positive correlation was observed between NLR and CRP levels (r = 0.393, p <0.001). The frequency with which ISR was encountered displayed a progressive and significant increase across tertile groups ( Figure 1 ). Patients in tertile 3 had significantly higher rates of ISR (p <0.001). Likewise, higher NLRs before stent deployment were found to be associated with an increased ISR by logistic regression analysis. Assuming NLR as a continuous (model 1) or a categorical (model 2) variable in multiple logistic regression analysis, smoking, diabetes mellitus, stent length, NLR, and CRP level emerged as independent predictors of ISR ( Table 2 ). Receiver operating characteristics curves explored the relation between preprocedural NLR and ISR. Area under the curve was 0.85 (95% confidence interval 0.81 to 0.90; p <0.001). Using a cut point of 2.73, preprocedural NLR predicted ISR with a sensitivity of 80% and specificity of 75% ( Figure 2 ). Mean NLR was found to be significantly increased in the restenosis group in patients with stable angina pectoris (n = 436, 2.07 ± 0.56 vs 3.48 ± 1.68, p <0.001) and unstable angina pectoris (n = 190, 2.73 ± 0.7 vs 4.56 ± 2.1, p <0.001) ( Figure 3 ).
| Variables | Univariable | Stepwise Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Neutrophil/lymphocyte ratio as continuous variable (model 1) | ||||
| Time between 2 coronary angiographies | 0.93 (0.91–0.95) | <0.001 | 0.99 (0.87–1.12) | 0.93 |
| Neutrophil/lymphocyte ratio | 1.33 (1.12–1.54) | <0.001 | 1.29 (1.16–1.43) | <0.001 |
| High-density lipoprotein | 0.97 (0.95–0.99) | <0.001 | 1.01 (0.95–1.06) | 0.78 |
| Low-density lipoprotein | 0.99 (0.99–1.00) | 0.47 | ||
| Triglycerides | 1.00 (0.99–1.02) | 0.20 | ||
| Left ventricular ejection fraction | 0.98 (0.95–1.01) | 0.31 | ||
| Diabetes mellitus | 1.23 (1.10–1.37) | 0.014 | 1.20 (1.09–1.32) | 0.022 |
| C-reactive protein | 1.19 (1.07–1.32) | 0.001 | 1.18 (1.09–1.68) | 0.01 |
| Stent length | 1.42 (1.03–1.86) | <0.001 | 1.35 (1.07–1.62) | <0.001 |
| Stent diameter | 1.15 (1.08–1.23) | 0.012 | 1.04 (0.95–1.14) | 0.22 |
| Current smoker | 1.59 (1.15–2.21) | 0.005 | 2.01 (0.84–5.08) | 0.021 |
| Neutrophil/lymphocyte ratio as categorical variable (model 2) | ||||
| Time between 2 coronary angiographies | 0.95 (0.92–1.01) | <0.001 | 0.99 (0.87–1.12) | 0.84 |
| Neutrophil/lymphocyte ratio >2.73 | 2.32 (1.51–3.83) | <0.001 | 1.85 (1.13–2.45) | <0.001 |
| High-density lipoprotein | 0.95 (0.91–1.02) | <0.001 | 1.03 (0.94–1.10) | 0.72 |
| Low-density lipoprotein | 0.93 (0.90–1.04) | 0.36 | ||
| Triglycerides | 0.98 (0.96–1.10) | 0.17 | ||
| Left ventricular ejection fraction | 1.01 (0.95–1.07) | 0.29 | ||
| Diabetes mellitus | 1.34 (1.16–1.51) | <0.001 | 1.27 (1.17–1.39) | <0.001 |
| C-reactive protein | 1.05 (0.93–1.46) | 0.002 | 1.14 (1.07–1.36) | 0.020 |
| Stent length | 1.56 (1.11–1.92) | <0.001 | 1.31 (1.04–1.57) | <0.001 |
| Stent diameter | 1.11 (1.03–1.18) | 0.024 | 1.01 (0.94–1.09) | 0.32 |
| Current smoker | 1.43 (1.05–2.06) | 0.005 | 1.93 (0.95–2.92) | 0.031 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


