The relation between left anterior descending coronary artery (LAD) anatomic features and clinical outcomes in patients with anterior ST-segment elevation myocardial infarction has not been fully investigated. The Intracoronary Abciximab and Aspiration Thrombectomy in Patients With Large Anterior Myocardial Infarction (INFUSE-AMI) trial randomized 452 patients with anterior ST-segment elevation myocardial infarctions who underwent mechanical revascularization to intralesional abciximab versus no abciximab and to manual thrombus aspiration versus no aspiration. The primary end point was infarct size (percentage left ventricular mass) on contrast magnetic resonance imaging at 30 days. “Wraparound LAD” was defined as an LAD reaching the apex and supplying the apical inferior aspect of the heart. Among complete data available in 338 patients, 258 (76.3%) had wraparound LADs. Global infarct size (17.4% vs 16.1%, p = 0.64) and the left ventricular ejection fraction (49.7% vs 48.7%, p = 0.98) by contrast magnetic resonance imaging at 30 days were comparable between patients with and those without wraparound LADs. Regional apical anterior infarct size was comparable (59.5% vs 55.8%, p = 0.559) between the groups; however, apical septal (61.3% vs 48.9%, p = 0.005), apical inferior (19.0% vs 3.7%, p <0.0001), and apical lateral (12.2% vs 4.8%, p = 0.0584) infarct sizes were larger in patients with wraparound LADs compared with those with nonwraparound LADs. The incidence of new-onset severe heart failure at 1 year was significantly higher in patients with compared with those without wraparound LADs (6.3% vs 0%, p = 0.02). In conclusion, in patients with anterior ST-segment elevation myocardial infarctions, as compared with the LAD not supplying the inferior aspect of the heart, a wraparound LAD was associated with a larger left ventricular apex infarct size, resulting in worse adverse events at 1 year.
Acute myocardial infarction is the most common cause of mortality in developed countries. Patients with anterior ST-segment elevation myocardial infarctions (STEMIs) that are caused by left anterior descending coronary artery (LAD) occlusions are at greatest risk for adverse clinical outcomes. LAD occlusion is one of the strongest determinates of infarct size, and an LAD that wraps around the left ventricular (LV) apex theoretically supplies a greater amount of myocardium than one that ends at or before the apex. Therefore, we used data from the Intracoronary Abciximab and Aspiration Thrombectomy in Patients With Large Anterior Myocardial Infarction (INFUSE-AMI) randomized clinical trial to examine the impact of LAD wrapping around the LV apex on infarct size and on clinical outcomes in patients with anterior STEMIs.
Methods
The INFUSE-AMI trial has been previously described in detail. In brief, 452 patients with anterior STEMIs and symptom onset–to–reperfusion time <5 hours who underwent primary percutaneous coronary intervention with bivalirudin anticoagulation were randomized in a 2 × 2 factorial design to (1) intralesional abciximab delivered locally at the site of the infarct lesion using the ClearWay RX catheter (Atrium Medical Corporation, Hudson, New Hampshire) versus no abciximab or to (2) thrombus aspiration using the Export catheter (Medtronic, Minneapolis, Minnesota) versus no thrombus aspiration. The primary end point of the study was infarct size measured at 30 days on contrast magnetic resonance imaging (cMRI) and expressed as percentage of LV mass in patients assigned to intralesional abciximab versus no abciximab, pooled across strata of aspiration. The secondary major end point was infarct size in patients assigned to aspiration versus no aspiration, pooled across strata of intralesional abciximab. All patients received aspirin plus clopidogrel or aspirin plus prasugrel. Among 452 patients in the INFUSE-AMI trial, 378 patients with available cMRI studies at 30 days were enrolled in the present substudy and were categorized into 2 groups on the basis of coronary angiography: (1) patients with LADs wrapping around the LV apex (wraparound LAD) and (2) patients with LADs not wrapping around the LV apex (nonwraparound LAD). Wraparound LAD was defined as an LAD reaching the apex and supplying the apical inferior aspect of the heart; a nonwraparound LAD was defined as an LAD terminating at or before the apex and not supplying the apical inferior aspect of the heart ( Figure 1 ). Whether the LAD wrapped around the LV apex to supply the apical inferior aspect of the heart was assessed by 2 independent cardiologists (NK, SJB) who were blinded to clinical or trial data; classification was based on agreement between the 2 investigators. In addition, all angiograms were reviewed in detail at an independent core laboratory (Cardiovascular Research Foundation, New York, New York) without knowledge of treatment assignment. Specifically, qualitative and quantitative coronary angiographic analysis using standard definitions was performed for Thrombolysis In Myocardial Infarction (TIMI) flow grade and myocardial blush grade at baseline, before stent implantation, and on final angiographic examination. Myocardial blush grade was assessed according to the semiquantitative densitometric method that evaluates the maximal intensity of contrast penetrating the infarct zone in comparison with unaffected territories.
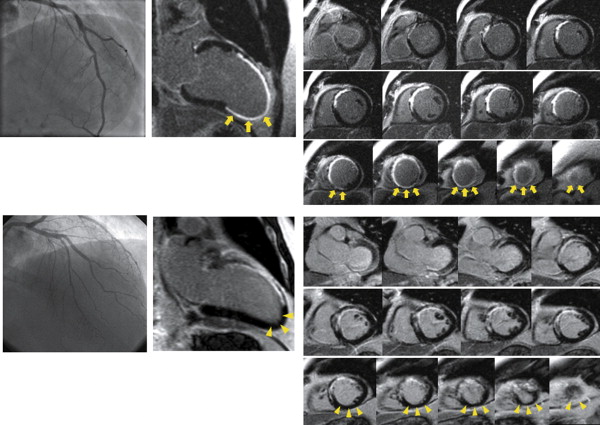
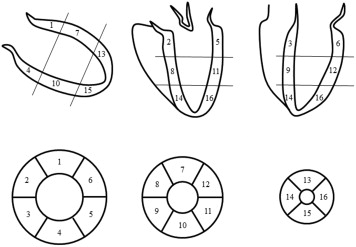
All contrast magnetic resonance images were acquired on a commercially available 1.5-T scanner. Each site was qualified by reviewing 2 recent cMRI cases performed using the imaging protocol prespecified for the trial. The cMRI examination consisted of 2 components: (1) cine cMRI for LV volume and systolic function and (2) delayed-enhancement cMRI for evaluation of infarct mass and total LV mass. All contrast magnetic resonance images were analyzed at an independent core laboratory (Cardiovascular Research Foundation) without knowledge of treatment assignment. cMRI analysis was performed using the US Food and Drug Administration–approved, multivendor-compatible ReportCard software version 4.0 (NeoSoft LLC, Waukesha, Wisconsin). LV volumes were determined by manually tracing the endocardial borders, excluding the papillary muscles, at end-diastole and end-systole on all short-axis cine contrast magnetic resonance images. LV mass (the area between the epicardial and endocardial borders) and infarct area (the white area within the black myocardium) were segmented on the delayed-enhancement contrast magnetic resonance images. Infarct size was calculated as infarct mass divided by total myocardial mass. LV was divided into 16 segments, and regional infarct size was calculated for each region ( Figures 1 and 2 ). Wall motion abnormalities were visually classified as normal (score 0), hypokinetic (score 1), akinetic (score 2), or dyskinetic (score 3) and were summed as total abnormal wall motion score.
Clinical outcomes, including death, reinfarction, ischemia-driven target vessel revascularization, new-onset severe heart failure, rehospitalization for heart failure, or stroke at 30 days and 1 year, were adjudicated by an independent clinical events committee. Major adverse cardiac events included death, reinfarction, new-onset severe heart failure, and rehospitalization for heart failure.
Baseline patient clinical characteristics, cMRI findings, and clinical outcomes were analyzed at a per patient level; angiographic findings were analyzed at a per lesion level. Categorical variables are summarized as frequencies and were compared between groups using chi-square or Fisher’s exact tests. Continuous variables are expressed as medians and interquartile ranges and were compared using Wilcoxon’s rank sum test. Time-to-event data are summarized as Kaplan-Meier estimates and were compared between groups with the log-rank test. Intra- and interobserver variability for the diagnosis of wraparound LAD was measured with the κ test of concordance. Statistical significance was set at p <0.05. Statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, North Carolina).
Results
Among 452 patients in the INFUSE-AMI trial, patients who did not undergo cMRI because of death within 30 days or other reasons were excluded. Of the 378 patients with cMRI data at 30 days, 40 (10.6%) were unable to be classified into the wraparound or nonwraparound LAD group because of poor angiographic quality (n = 37) or postprocedural TIMI flow grade 0 or 1 (n = 3, because the distal LAD was not visualized). Among the remaining 338 patients, 258 (76.3%) had wraparound LADs, and 80 (23.7%) had nonwraparound LADs. Interobserver (NK and SJB) and intraobserver (NK, 4 months apart) variability values to categorize wraparound versus nonwraparound LAD were 0.84 and 0.84, respectively.
Baseline clinical features of the 2 groups are listed in Table 1 . Baseline angiographic findings are listed in Table 2 . Multivessel disease was more frequently observed in patients with wraparound LADs (p = 0.04). Culprit lesions were more frequently located within the proximal LAD in patients with nonwraparound LADs (p = 0.04). The frequency of initial TIMI flow grade 0 or 1 was 68.6% in patients with wraparound LADs and 80.0% in patients with nonwraparound LADs (p = 0.05).
| Variable | Wrap-around Left Anterior Descending Artery | p Value | |
|---|---|---|---|
| Yes n = 258 | No n = 80 | ||
| Age (years) | 60.0 [51.0, 69.0] | 57.0 [50.5, 66.5] | 0.31 |
| Male | 74.4% (192/258) | 73.8% (59/80) | 0.90 |
| BMI (kg/m 2 ) | 26.2 [23.9, 29.4] | 27.3 [24.8, 30.3] | 0.13 |
| Hypertension | 30.2% (78/258) | 25.0% (20/80) | 0.37 |
| Hyperlipidemia | 14.7% (38/258) | 16.5% (13/79) | 0.71 |
| Diabetes mellitus | 7.4% (19/258) | 13.8% (11/80) | 0.08 |
| Current smoking | 48.2% (124/257) | 40.5% (32/79) | 0.23 |
| Renal insufficiency | 1.2% (3/258) | 1.3% (1/80) | 1.00 |
| Prior PCI | 1.6% (4/257) | 1.3% (1/80) | 1.00 |
| Peripheral vascular disease | 1.2% (3/258) | 1.3% (1/79) | 1.00 |
| Killip classification | |||
| I | 87.5% (225/257) | 78.8% (63/80) | 0.051 |
| II | 7.0% (18/257) | 7.5% (6/80) | 0.88 |
| III | 0.4% (1/257) | 1.3% (1/80) | 0.42 |
| Peak creatine kinase (U/mL) | 2170 [979, 4040] | 2022 [794, 3853] | 0.41 |
| Peak creatine kinase MB (U/mL) | 235 [130, 392] | 262 [130, 462] | 0.53 |
| Medication at the discharge | |||
| Beta blocker | 95.7% (247/258) | 100.0% (80/80) | 0.07 |
| ACE inhibitor or ARB | 93.8% (242/258) | 93.8% (75/80) | 1.00 |
| Statin | 99.2% (256/258) | 98.8% (79/80) | 0.56 |
| Variable | Wrap-around Left Anterior Descending Artery | p Value | |
|---|---|---|---|
| Yes | No | ||
| Procedure summary | 258 patients | 80 patients | |
| Symptom onset to first device (min) | 151 [121, 211] | 155 [116, 220] | 0.73 |
| Aspiration catheter use | 51.2% (132/258) | 55.0% (44/80) | 0.55 |
| ClearWay RX catheter used | 51.6% (133/258) | 45.0% (36/80) | 0.31 |
| DES implanted | 74.8% (193/258) | 82.5% (66/80) | 0.16 |
| Maximum stent or balloon size (mm) | 3.5 [3.0, 3.5] | 3.0 [3.0, 3.5] | 0.29 |
| Angiographic findings before PCI | 260 lesions | 81 lesions | |
| Multivessel disease | 40.3% (104/258) | 27.5% (22/80) | 0.04 |
| Culprit lesion located in proximal LAD | 59.7% (154/258) | 72.5% (58/80) | 0.04 |
| Lesion length (mm) | 14.35 [11.33, 21.10] | 12.89 [10.45, 17.03] | 0.04 |
| Diameter stenosis (%) | 100.0 [84.1, 100.0] | 100.0 [87.7, 100.0] | 0.37 |
| TIMI flow grade | |||
| 0 or 1 | 68.6% (177/258) | 80.0% (64/80) | 0.05 |
| 2 | 17.1% (44/258) | 8.8% (7/80) | 0.07 |
| 3 | 14.3% (37/258) | 11.3% (9/80) | 0.48 |
| Thrombus | 86.2% (224/260) | 84.0% (68/81) | 0.62 |
| Collaterals | 26.9% (70/260) | 30.9% (25/81) | 0.49 |
| ACC/AHA classification type B2/C | 93.8% (244/260) | 93.8% (76/81) | 1.00 |
| Angiographic findings after PCI | 260 lesions | 81 lesions | |
| Residual thrombus | 1.2% (3/260) | 2.5% (2/81) | 0.34 |
| Stented lesion length (mm) | 21.82 [17.47, 28.00] | 22.16 [17.28, 29.20] | 0.80 |
| TIMI flow grade | |||
| 0 or 1 | 0.8% (2/258) | 1.3% (1/80) | 0.56 |
| 2 | 5.4% (14/258) | 10.0% (8/80) | 0.15 |
| 3 | 93.8% (242/258) | 88.8% (71/80) | 0.13 |
| MBG | 0.8% (2/258) | 1.3% (1/80) | 0.56 |
| 0 or 1 | 17.8% (46/258) | 16.3% (13/80) | 0.75 |
| 2 or 3 | 82.2% (212/258) | 83.8% (67/80) | |
Table 3 lists the global LV cMRI findings at 30 days. Total infarct size, the LV ejection fraction, LV end-diastolic volume index, LV end-systolic volume index, and total abnormal wall motion score were comparable between the 2 groups at 30 days.
| Variable | Wrap-around Left Anterior Descending Artery | p Value | |
|---|---|---|---|
| Yes n = 258 | No n =80 | ||
| Total left ventricular myocardial mass (g) | 128.7 [108.0, 154.7] | 130.5 [107.9, 152.3] | 0.94 |
| Infarct mass (g) | 21.7 [10.8, 33.9] | 19.5 [8.4, 31.8] | 0.50 |
| Infarct size (%) | 17.4 [8.7, 23.5] | 16.1 [6.8, 24.6] | 0.64 |
| Left ventricular ejection fraction (%) | 49.7 [42.7, 57.0] | 48.7 [43.3, 57.7] | 0.98 |
| Left ventricular end diastolic volume index (mL/m 2 ) | 90.8 [75.6, 103.9] | 85.7 [77.4, 99.4] | 0.52 |
| Left ventricular end systolic volume index (mL/m 2 ) | 44.7 [33.8, 58.3] | 43.6 [32.9, 54.3] | 0.69 |
| Total abnormal wall motion score | 8.0 [2.0, 10.0] | 7.0 [1.5, 10.0] | 0.28 |
Table 4 lists regional infarct size at 30 days. Apical anterior infarct size was comparable between the 2 groups (median 59.5% vs 55.8%, p = 0.56). However, apical septal (median 61.3% vs 48.9%, p = 0.005), apical inferior (median 19.0% vs 3.7%, p <0.0001), and apical lateral (median 12.2% vs 4.8%, p = 0.058) infarct sizes were larger in patients with wraparound LADs than those with nonwraparound LADs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


