Atrial fibrillation (AF) is the most common cardiac arrhythmia in adults and has been independently related to increased morbidity and mortality. AF is a frequent arrhythmia in infective endocarditis (IE). Nevertheless, there are no data on how AF affects the clinical outcome of patients with endocarditis. Our purpose was to investigate patient characteristics, microbiology, echocardiographic findings, in-hospital course, and prognosis of patients with IE who develop new-onset AF (NAF) and compare them with those who remained in sinus rhythm (SR) or had previous AF (PAF). From 1997 to 2014, 507 consecutive patients with native left-sided IE were prospectively recruited at 3 tertiary care centers. We distinguished 3 groups according to the type of baseline heart rhythm during hospitalization and previous history of AF: NAF group (n = 52), patients with no previous history of AF and who were diagnosed as having NAF during hospitalization; SR group (n = 380), patients who remained in SR; and PAF group (n = 75), patients with PAF. Patients with NAF were older than those who remained in SR (68.3 vs 59.6 years, p <0.001). At admission, heart failure was more common in NAF group (53% vs 34.3%, p <0.001), whereas stroke (p = 0.427) was equally frequent in all groups. During hospitalization, embolic events occurred similarly (p = 0.411). In the multivariate analysis, NAF was independently associated with heart failure (odds ratio 3.56, p <0.01) and mortality (odds ratio 1.91, p = 0.04). In conclusion, the occurrence of NAF in patients with IE was strongly associated with heart failure and higher in-hospital mortality independently from other relevant clinical variables.
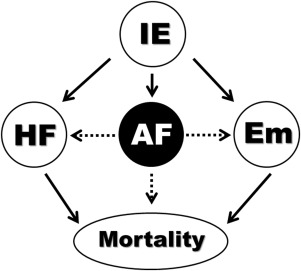
Atrial fibrillation (AF) is the most common cardiac arrhythmia in adults and has been independently related to increased morbidity and mortality. It is also well known the association between new-onset AF (NAF) and mortality in patients hospitalized with severe sepsis or heart failure. Systemic inflammation and hemodynamic disorders have been involved in the origin and perpetuation of AF in these clinical scenarios. Infective endocarditis (IE) is a severe infectious disease producing a great systemic inflammatory reaction and local valve destruction leading to severe hemodynamic changes. Not surprisingly, AF is common in IE, sometimes coexisting with heart failure and systemic embolism. Nevertheless, there are no data on how AF affects the clinical outcome of patients with IE. We hypothesized that patients with IE and NAF have a worse clinical outcome than those who remain in sinus rhythm (SR). Patients with NAF might be particularly prone to congestive heart failure and thromboembolic events ( Figure 1 ). The purpose of this study was to investigate patient characteristics, microbiology, and echocardiographic findings of patients with IE and NAF. We further aim to compare the in-hospital course and prognosis of patients who develop de novo AF with that of those who remained in SR or had previous AF (PAF).
Methods
This study was conducted at 3 tertiary care centers with surgical facilities, which have been working together on IE with the use of standardized protocols. To ensure consecutive enrollment, all patients who underwent echocardiography to rule out IE were clinically followed until a diagnosis was established. Only definite cases of left-sided IE were included. Duke criteria were applied until 2002 and modified Duke criteria thereafter. Right-sided episodes were excluded because of their different epidemiology, clinical characteristics, and prognosis.
From 1997 to 2014, 926 consecutive patients with left-sided IE were prospectively recruited on an ongoing multipurpose database, 110 patients (11.9%) of them were excluded from the analysis because of inability to assess the baseline heart rhythm during hospitalization. Patients with prosthetic valve endocarditis were also excluded from the study (309 patients). The remaining 507 patients with native left-sided IE formed our final study population.
This registry complies with the Declaration of Helsinki and was approved by the local ethical committee. All participants gave written informed consent. The proportion of missing data was <10% in all analyzed variables. For purposes of analysis and comparison, we distinguished 3 groups according to the type of baseline heart rhythm during hospitalization and previous history of AF: NAF group (n = 52), patients with no previous history of AF and who were diagnosed as having NAF during hospitalization; SR group (n = 380), patients without a history of PAF, who remained in SR and who did not suffer AF during hospitalization; and PAF group (n = 75) included patients with PAF (permanent, paroxysmal, or persistent). Those patients who developed postoperative AF after surgery for IE were not considered as NAF.
All patients underwent transthoracic and transesophageal echocardiography. A set of 3 blood cultures was obtained at admission and 3 additional blood cultures 48 to 72 hours later. If blood cultures were negative after 72 hours, specific serologic tests were done for Chlamydia , Brucella , Q fever, Legionella , Mycoplasma , and Bartonella .
Nosocomial and community-acquired IE were defined according to the study. Acute onset IE was applied when the time between the appearance of symptoms and hospital admission was <15 days. Previous valvulopathy was defined as any kind of valvular heart disease and congenital valvular disease. Anemia was defined as a hemoglobin concentration below 9 g/dl; renal insufficiency was established when the serum creatinine concentration was >2 mg/dl. Heart failure was diagnosed according to Framingham criteria. Under the term of immunosuppression were included patients with human immunodeficiency virus and those who were on steroids or other immunosuppressive therapy. Persistent signs of infection and septic shock were defined as previously described. The diagnosis of systemic embolism was based on clinical signs and data derived from imaging procedures.
NAF was defined when AF was registered in a 12-lead electrocardiogram done during hospitalization in a patient with previously documented SR and no previous history of AF. Paroxysmal, persistent, and permanent AF were defined according to the guidelines.
The echocardiographic criteria used for definition and measurement of vegetations, abscesses, pseudoaneurysms, and fistulas have been described elsewhere. Left atrial dimension was measured in M mode and 2-dimensional transthoracic echocardiography after the recommendations of the American Society of Echocardiography.
Surgery was defined as early if done before antibiotic treatment was completed and was performed when any of the following occurred: refractory heart failure, recurrent embolism with persistent vegetations in the echocardiogram, persistent signs of infection, and fungal endocarditis. When a patient meeting surgical criteria did not undergo surgery, the reason was either because of patient rejection, unacceptably high surgical risk, or when the patient was too frail.
Continuous variables are reported as a mean value and SD or median and interquartile range in cases of nonnormality. Continuous variables were compared between the groups with a 2-tailed Student t test or Mann–Whitney U test when necessary. Categorical variables are expressed as a frequency and percentage and were compared with the chi-square test and Fisher’s exact test when appropriate. In case of multiple categories, analysis of variance or Kruskal–Wallis test were used.
Two multivariate logistic regression analyses were performed, one for prediction of mortality and another for detection of independent factors for heart failure. We included in the model the variables previously known to be associated to these events and those considered clinically relevant. When a variable statistically significant in the univariate analysis was not included in the multivariate analysis, the reason was collinearity or absence of change in the effect of AF. In addition, interactions between variables included in the model were assessed in the model. The adjusted odds ratios (ORs) with 95% CIs for each variable have been calculated. All test were 2-tailed, and the differences were considered statistically significant at p values <0.05. Statistical analysis was performed with PASW Statistics, version 17.0, (SPSS Inc. Chicago, Illinois).
Results
Demographic characteristics, co-morbidities, and clinical presentation comparisons between groups are summarized in Table 1 . Patients with NAF were older than those who remained in SR. Concerning co-morbidities, chronic renal failure, and chronic obstructive pulmonary disease were more common in NAF group than in SR group. At admission, heart failure and their radiological manifestations were more common in NAF group, whereas stroke and systemic embolism were equally present in all groups. Interestingly, blood levels of acute phase reactants at admission (C-reactive protein) were higher in patients with NAF ( Table 1 ).
| NAF (n=52) | SR (n=380) | PAF (n= 75) | p | |
|---|---|---|---|---|
| Age (years) | 68.3 (10.2) | 59.6 (16.2) | 68.9 (12.1) | <0.001 ∗ ‡ |
| Male | 36 (69%) | 259 (68.2%) | 44 (59%) ‡ | 0.260 |
| Community-acquired IE | 33 (64%) | 297 (78.6%) | 44 (59%) | 0.002 ∗ ‡ |
| Previous valvulopathy | 27 (54%) | 154 (43.6%) | 59 (80%) | <0.001 † ‡ |
| Anemia | 12 (23%) | 76 (20.2%) | 23 (31%) | 0.135 ‡ |
| Chronic renal failure | 10 (19%) | 34 (9%) | 11 (15%) | 0.047 ∗ |
| Diabetes | 15 (29%) | 67 (17.8%) | 20 (27%) | 0.057 ∗ |
| Alcoholism | 6 (12%) | 41 (10.9%) | 7 (9.3%) | 0.903 |
| Chronic obstructive pulmonary disease | 7 (14%) | 20 (5.3%) | 11 (15%) | 0.005 ∗ ‡ |
| Malignant neoplasia | 6 (12%) | 43 (11.4%) | 11 (15%) | 0.721 |
| Immunosuppression | 4 (8%) | 31 (8.2%) | 8 (11%) | 0.776 |
| Symptoms to admission (days) | 12.5 (7-57) | 20.5 (7-60) | 20 (7-35) | 0.497 |
| Acute onset (<15days) | 26 (50%) | 145 (38.7%) | 33 (44%) | 0.241 |
| Fever at admittance | 32 (63%) | 281 (75.5%) | 53 (72%) | 0.138 |
| Heart failure | 27 (53%) | 129 (34.3%) | 38 (51%) | 0.003 ∗ ‡ |
| Acute renal failure | 16 (31%) | 73 (19.5%) | 13 (17%) | 0.131 ∗ |
| Septic shock | 6 (12%) | 17 (4.6%) | 7 (9%) | 0.108 ∗ |
| Chest pain | 5 (10%) | 38 (10.2%) | 7 (9%) | 0.968 |
| Abdominal pain | 5 (10%) | 43 (11.6%) | 6 (8%) | 0.631 |
| Splenomegaly | 4 (8%) | 39 (10.5%) | 4 (5%) ‡ | 0.063 |
| Confusional syndrome | 11 (21%) | 52 (13.9%) | 12 (16%) | 0.376 |
| Coma | 2 (4%) | 11 (2.9%) | 2 (3%) | 0.923 |
| Stroke | 0.427 | |||
| Hemorrhagic | 1 (2%) | 13 (3.5%) | 2 (3%) | |
| Ischemic | 7 (14%) | 47 (12.5%) | 4 (5%) | |
| Systemic embolism | 9 (17%) | 85 (22.5%) | 11 (15%) | 0.248 |
| Hematuria | 4 (8%) | 14 (3.7%) | 1 (1%) | 0.181 |
| Arthritis/Spondylodiscitis | 5 (10%) | 66 (17.7%) | 6 (8%) | 0.052 ‡ |
| Anticoagulation | 11 (24.4%) | 23 (6.2%) | 53 (71%) | <0.001 † ‡ |
| Second and third degree AV block | 2 (4%) | 8 (2.1%) | 1 (1%) | 0.646 |
| Left bundle-branch block | 3 (6%) | 10 (2.7%) | 7 (10%) | 0.017 ‡ |
| Cardiomegaly | 37 (71%) | 160 (42.4%) | 50 (69%) | <0.001 ∗ ‡ |
| Pleural effusion | 22 (43%) | 83 (22.1%) | 19 (26.4%) | 0.005 ∗ † |
| C-reactive protein (mg/dl) § | 17.9 (6.9-115.3) | 14.3 (4.8-67.8) | 6.9 (2.9-14.8) | 0.026 ∗ † ‡ |
| Hemoglobin (g/dl) | 11 (2.2) | 11.1 (2.1) | 11 (2) | 0.955 |
| Platelets | 228×10 3 (124×10 3 ) | 215×10 3 (141×10 3 ) | 196×10 3 (99×10 3 ) ‡ | 0.114 |
∗ Statistically significant differences between NAF-group and SR-group (p <0.05).
† Statistically significant differences between NAF-group and PAF-group (p <0.05).
‡ Statistically significant differences between SR-group and PAF-group (p <0.05).
The microorganisms found in patients with NAF were not significantly different from those isolated in SR group and PAF group ( Table 2 ).
| NAF (n=52) | SR-Group (n=380) | PAF-Group (n= 75) | p | |
|---|---|---|---|---|
| Streptococcus bovis | 2 (4%) | 23 (6.1%) | 3 (4%) * | 0.654 |
| Streptococcus viridans | 5 (10%) | 69 (18.3%) | 6 (8%) | 0.036 * |
| Other estreptococci | 4 (8%) | 32 (8.5%) | 5 (7%) | 0.864 |
| Enterococci | 6 (11.5%) | 33 (8.8%) | 8 (11%) | 0.738 |
| Staphylococcus aureus | 7 (14%) | 66 (17.5%) | 13 (17%) | 0.766 |
| Coagulase negative staphylococci | 10 (19%) | 45 (11.9%) | 13 (17%) | 0.202 |
| Gram negative bacilli | 1 (2%) | 11 (2.9%) | 2 (3%) | 0.918 |
| Fungi | 0 (0%) | 4 (1.1%) | 2 (3%) | 0.355 |
| HACEK Group | 0 (0%) | 4 (1.1 %) | 0 (0%) | 0.507 |
| Anaerobes | 0 (0%) | 3 (0.8%) | 1 (1%) | 0.707 |
| Polymicrobial | 3 (6%) | 20 (5.3%) | 7 (9%) | 0.403 |
| Others | 2 (4%) | 13 (3.4%) | 4 (5%) | 0.736 |
| Negative cultures | 12 (23.1%) | 54 (14.3%) | 11 (15%) | 0.255 |
* Statistically significant differences between SR group and PAF group (p <0.05).
Echocardiographic data are presented in Table 3 . As expected, left atrial dimension was larger in patients with NAF than in those who remained in SR. Vegetations were equally present in groups ( Table 3 ). Vegetation size was similar in all groups. As regard to the presence of moderate-to-severe valvular insufficiency, no differences were found between patients with NAF and those who remained in SR ( Table 3 ).
| NAF (n=52) | SR (n=380) | PAF (n= 75) | p | |
|---|---|---|---|---|
| Location of the infection | ||||
| Aortic native valve | 29 (56%) | 216 (56.8%) | 39 (52%) | 0.742 |
| Mitral native valve | 30 (58%) | 227 (59.7%) | 45 (60%) | 0.958 |
| Left atrial dimension (mm) | 48 (8.5) ∗ | 43.3 (6.8) | 52.4 (14.3) | <0.001 ∗ ‡ |
| Vegetations | ||||
| Detection by echocardiography | 48 (98%) | 332 (90%) | 61 (84%) | 0.035 † |
| Vegetation size (mm) | 12 (9.4-18.3) | 12.7 (9-18.5) | 12 (7-18·4) | 0.805 |
| Moderate-severe valvular regurgitation | 40 (82%) | 314 (85.1%) | 55 (75%) | 0.118 ‡ |
| Periannular complications | 14 (29%) | 92 (24.9%) | 15 (21%) | 0.582 |
| Abscess | 9 (16%) | 46 (12.5%) | 14 (19%) | 0.275 |
| Pseudoaneurysm | 9 (18%) | 61 (16.5%) | 6 (8%) ‡ | 0.168 |
| Fistula | 1 (2%) | 9 (2.4%) | 1 (1%) | 0.849 |
∗ Statistically significant differences between NAF-group and SR-group (p <0.05).
† Statistically significant differences between NAF-group and PAF-group (p <0.05).
‡ Statistically significant differences between SR-group and PAF-group (p <0.05).
Heart failure was significantly more frequent in patients with NAF ( Table 4 ). In this group, 27 patients (53%) were already in heart failure at admission. Among patients with heart failure, moderate-to-severe valvular insufficiency was more frequently encountered in patients with SR than in those with AF irrespective of the type (NAF or PAF) (NAF group: 34 (85%), SR group: 193 (93.7%), and PAF group: 42 (82.4%); p = 0.020). According to these results, patients with heart failure and NAF underwent surgery less frequently than those with heart failure and SR (NAF group: 20 [48.8%], SR group: 146 [68.5%], and PAF group: 33 [62.3%]; p = 0.048).
| NAF (n=52) | SR (n=380) | PAF (n= 75) | p | |
|---|---|---|---|---|
| Heart failure | 41 (79%) | 219 (58.4%) | 53 (71%) | 0.005 ∗ |
| CNS embolism | 7 (14%) | 50 (13.3%) | 5 (7%) | 0.373 |
| Systemic embolism | 15 (29%) | 123 (32.8%) | 19 (25%) | 0.411 |
| Acute renal insufficiency | 28 (54%) | 178 (47.3%) | 45 (60%) | 0.113 † |
| Septic shock | 13 (25%) | 58 (15.7%) | 17 (23%) | 0.122 |
| Cardiac surgery | 26 (50%) | 219 (57.6%) | 40 (53%) | 0.502 |
| Death | 24 (48%) | 95 (25.6%) | 27 (37%) | 0.002 ∗ † |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


