The outcome of biventricular (BV) repair for right-dominant unbalanced atrioventricular canal has remained poor, because it is difficult to predict left ventricular (LV) adequacy before surgery. Our aim was to determine whether preoperative echocardiographic parameters, specifically analysis of color inflow into the LV, would predict survival after BV repair in patients with right-dominant unbalanced atrioventricular canal. Subjects with right-dominant unbalanced atrioventricular canal diagnosed from 1994 to 2007 were included. The echocardiographic parameters were analyzed blinded to the palliation strategy and survival. The LV inflow index (LVII) was calculated as the secondary color inflow diameter indexed to the left atrioventricular valve (AVV) annulus diameter. Univariate analysis, survival analysis, and multivariate modeling with stepwise logistic regression were performed. Of the 45 subjects, 23 (51%) underwent single ventricle (SV) palliation and 22 (49%) underwent BV repair. Of the 23 who underwent SV palliation, 15 (65%) survived compared to 18 (82%) of 22 who underwent BV repair (p = 0.34). In the BV group, a greater LVII predicted survival (R2 = 0.46, p = 0.03). No subjects with a LVII <0.5 survived BV repair. Mortality in the BV group was associated with younger age at initial surgery (p <0.01) and abnormal left AVV morphology (p = 0.02). Of the BV subjects with a patent ductus arteriosus at the initial operation (n = 11), the nonsurvivors were more likely to have retrograde flow in the transverse arch (p <0.01). In the BV group, reoperation within 30 days of the initial repair was strongly associated with mortality (p <0.01). In conclusion, in cases of mild or moderate LV hypoplasia, a greater LVII predicted survival after BV repair in patients with right-dominant unbalanced atrioventricular canal. We propose incorporation of the LVII into the echocardiographic assessment of these patients.
In unbalanced atrioventricular canal defect, the common atrioventricular valve (AVV) is positioned disproportionately over one ventricle, with variable degrees of hypoplasia of the contralateral ventricle. When unbalanced to the right ventricle, the left ventricle might be inadequate to support the systemic circulation. The surgical outcome for right-dominant unbalanced atrioventricular canal remains poor because of the difficulty in predicting whether a biventricular (BV) repair will be successful. Investigators have proposed various echocardiographic criteria to assess the left ventricular (LV) adequacy in patients with right-dominant unbalanced atrioventricular canal. van Son et al suggested that a preoperative LV potential volume >15 ml/m 2 predicts a favorable outcome for BV repair. Cohen et al used the proportion of the common AVV allocated to each ventricle to predict successful BV repair. In subjects with a borderline LV, multiple studies have highlighted the importance of the mitral valve annulus when considering BV repair. We hypothesized that adequate inflow into the LV would be similarly important in cases of right-dominant unbalanced atrioventricular canal. We devised a novel echocardiographic parameter, the LV inflow index (LVII), to evaluate the inflow into the LV and to predict whether BV repair will be successful.
Methods
The present study was a single-institution, retrospective case series. The institutional review board approved the study. The surgical database from January 1994 to September 2007 was reviewed. All subjects with an echocardiographic diagnosis of a right-dominant unbalanced atrioventricular canal, according to criteria previously described by Cohen et al, were included. Those with heterotaxy syndrome, dextrocardia, and/or conotruncal abnormalities, such as tetralogy of Fallot or a double-outlet right ventricle, were excluded. Demographic information for subjects was collected from chart review at the initial echocardiogram and at the definitive operation.
The subjects were stratified according to the initial surgical intervention. Those deemed to have severe LV hypoplasia or an AVV index <0.27 underwent single ventricle (SV) palliation. In contrast, subjects deemed to have mild LV hypoplasia underwent BV repair. However, the decision to proceed with SV or BV repair was ultimately at the discretion of the treating physicians. The SV group included all subjects who underwent a stage 1 Norwood procedure or a variation of that procedure as the initial surgical palliation. The BV group included all subjects who underwent a procedure involving patch or suture closure of the atrial and ventricular septal components or aortic arch repair as the initial surgical procedure, with the intention of septating the heart as a second procedure. The cardiopulmonary bypass times, cross-clamp times, and circulatory arrest times were recorded for each subject. The details of each operation, including the need for coarctation repair, were recorded.
All echocardiographic studies were performed using a Philips ultrasound system (Andover, Massachusetts) coupled with an appropriately sized transducer for the best quality image. The studies were stored on videotapes and analyzed using the Philips 5500 off-line cardiovascular analysis software package (Andover, Massachusetts). The echocardiographic parameters measured on the preoperative echocardiograms were assessed, blinded to the surgical palliation strategy and outcome. For all quantitative variables, 3 measurements were made and the results averaged. Two-dimensional measurements were made in the standard views. The z-scores, adjusted for body surface area for the aortic annulus, transverse arch, and isthmus were generated (unpublished data, Boston Children’s Hospital). The left, right, and total AVV areas were measured in the subcostal left anterior oblique plane, as previously described ; the left and right AVV annuli were measured in the apical 4-chamber view in diastole from the ventricular septum to the inner edge of the annulus. The ventricular septal defect and LV and right ventricular length were measured in the apical 4-chamber view at end-diastole (from the AVV annulus to the apex). The degree of regurgitation of the left and right AVVs were subjectively quantified as either mild or less or moderate or greater regurgitation. The LV volumes were calculated according to a single-plane area-length method using the apical 4-chamber view. The direction of flow at the transverse arch was categorized as either all anterograde or any retrograde flow in the cardiac cycle. The flow at the patent ductus arteriosus was noted as either all left to right or any right to left flow in the cardiac cycle. The architecture of the left-sided component of the common AVV was assessed in the parasternal short-axis view as either normal (2 adequately spaced papillary muscles) or abnormal (defined as 2 papillary muscles with 1 dominant and 1 hypoplastic papillary muscle, a single papillary muscle, or shortened chordae tendinae).
The primary and secondary inflow into the LV using color Doppler was measured in the 4-chamber view in diastole. The primary inflow was measured at the level of the left AVV annulus, and the secondary inflow was measured as the color inflow jet diameter at the level of the papillary muscles. An echocardiographic parameter, the LVII, was developed to assess inflow into the left ventricle. It was defined as the secondary inflow diameter into the left ventricle using color Doppler divided by the 2-dimensional left AVV annulus diameter ( Figure 1 ).
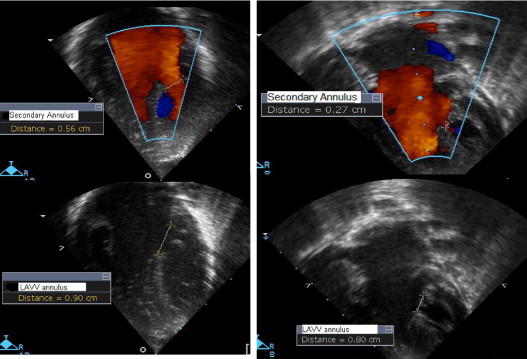
The medical records of all subjects were reviewed for the demographic data and to determine the clinical outcomes, including survival, the need for reoperation within 30 days of definitive repair, and the need for extracorporeal membrane oxygenation. The date of the most recent follow-up evaluation was recorded for survival analysis. Any genetic syndrome documented in the medical record was noted.
Statistical analysis was performed using Intercooled Stata, version 9 (StataCorp, College Station, Texas), and Statistical Package for Social Sciences, versions 15 and 17 (SPSS, Chicago, Illinois). Normally distributed data are reported as the mean ± SD. Dichotomous variables were tabulated. The subjects were divided according to the initial surgical strategy (i.e., SV palliation vs BV repair). A subgroup analysis was performed between the survivors and nonsurvivors of BV repair. The Wilcoxon rank-sum test was used to assess differences between the surgical groups for the continuous echocardiographic measurements. Fisher’s exact test was used to detect differences in the direction of the transverse arch flow, degree of AVV regurgitation, AVV morphology, underlying genetic syndrome, need for extracorporeal membrane oxygenation, need for coarctation repair, and need for reoperation. The Mann-Whitney U test was used to assess for any differences in the anatomic subtype type of canal defect between the SV and BV subjects. Univariate analysis for echocardiographic variables and multivariate modeling with stepwise logistic regression analysis were performed to determine which variables most strongly predicted survival. Variables with p <0.20 on univariate and multivariate analyses were included in the stepwise logistic regression analyses. For the univariate and multivariate analyses, p <0.05 was considered significant. The survival analysis was performed by generating Kaplan-Meier survival curves. For the purposes of the present analysis, an event was defined as either death or the requirement for heart transplantation. Comparisons between groups were performed using the Breslow test. The Spearman rank correlation test was used to evaluate for any significant associations between variables.
Results
A total of 45 subjects met the inclusion criteria. The median age at presentation was 1 day for the SV group and 17.5 days for the BV group. The median age at surgery was 4 days for the SV group and 55 days for the BV group. Current follow-up data were available for 73% of all subjects: 18 of 23 in the SV cohort and 15 of 22 in the BV cohort. In the SV cohort, 2 subjects (9%) had genetic syndromes identified (i.e., Ellis-van-Crevald and CHARGE [coloboma, heart defect, atresia choanae (also known as choanal atresia), retarded growth and development, genital abnormality, and ear abnormality] syndromes). In the BV cohort, 6 subjects (27%) were identified with genetic syndromes; all 6 had trisomy 21 (p = 0.13). None of the subjects in the BV repair group with trisomy 21 died.
Figure 2 illustrates the surgical history for the 23 subjects in the SV group. All subjects underwent an initial stage 1 surgery. In the SV cohort, 8 events (35%) occurred, 7 deaths and 1 heart transplant. During the initial hospitalization, 3 early deaths occurred within 30 days of initial palliation and 1 late death occurred after shunt thrombosis requiring extracorporeal membrane oxygenation. One subject underwent transplantation for ventricular dysfunction and AVV regurgitation requiring 2 separate episodes of extracorporeal membrane oxygenation. The survival to hospital discharge rate with a SV circulation was 78% (18 of 23). One subject was subsequently lost to follow-up after Norwood palliation, and there was one interstage death. Sixteen patients underwent the bidirectional Glenn operation. Of these, 1 subject underwent concurrent atrioventricular valvuloplasty and subsequently died. The final subject, with an underlying diagnosis of CHARGE syndrome, died before Fontan completion from a viral illness associated with respiratory failure. At the latest follow-up, 11 subjects (48%) had undergone the Fontan operation and 1 subject remained with bidirectional Glenn circulation.
Figure 2 also illustrates the surgical procedures for the 22 subjects in the BV repair group. Of the 22, 7 had an additional diagnosis of coarctation of the aorta. In the BV repair cohort, 4 subjects died. One subject underwent an isolated neonatal coarctation repair and subsequently developed low cardiac output secondary to residual LV outflow tract obstruction. This patient underwent conversion to SV palliation and subsequently died. Two subjects underwent simultaneous neonatal BV repair and coarctation repair. One of these had severe AVV regurgitation and residual coarctation. The patient underwent 2 reoperations and ultimately died. The second patient developed pulmonary hypertension postoperatively because of inadequate left-sided structures. The patient underwent conversion to a Norwood procedure and ultimately died. The final patient who did not survive BV repair hemorrhaged after the initial operation, requiring mediastinal exploration. Postoperatively, the patient had a low cardiac output and subaortic obstruction from AVV tissue and ultimately died.
Figure 3 demonstrates the Kaplan-Meier survival curve for the SV and BV cohorts. Survival was not significantly different statistically between the 2 groups: 15 (65%) of 23 for those who underwent SV palliation and 18 (82%) of 22 for those who underwent BV repair (p = 0.34). No significant difference was found in mortality among the anatomic subtype of AVC (incomplete, transitional, or complete forms; p = 0.33). Table 1 summarizes the operative and echocardiographic variables for the SV palliation and BV repair subjects. As expected, those who underwent SV palliation were younger (p <0.01) with a smaller body surface area (p = 0.005) at the initial operation. Using the 2-dimensional measurements, the z-scores for the left-sided structures were smaller in the SV population than in the BV repair group. The primary (p <0.001) and secondary (p = 0.001) inflows into the left ventricle by color Doppler were also significantly smaller statistically. The left/right AVV index was significantly smaller statistically in the SV subjects than in the BV subjects (p <0.001). Compared to those who underwent BV repair, those who underwent SV surgery were more likely to have retrograde flow in the transverse arch (p = 0.03) and more likely to require surgery for coarctation of the aorta (p <0.001). No statistically significant difference was found in the LVII in the SV population compared to the BV population (p = 0.72).
| Variable | SV (n = 23) | BV (n = 22) | p Value |
|---|---|---|---|
| Age at first study (days) | 1 (0–131) | 17.5 (0–287) | 0.005 ⁎ |
| Age at first surgery (days) | 4 (1–133) | 55 (3–322) | <0.001 ⁎ |
| Body surface area at first study (m 2 ) | 0.21 ± 0.03 | 0.25 ± 0.05 | 0.007 ⁎ |
| Weight at first surgery (kg) | 3.0 (1.4–4.8) | 3.95 (2.5–6.8) | 0.004 ⁎ |
| Cardiopulmonary bypass time (minutes) | 80 (70–176) | 71 (37–136) | 0.002 ⁎ |
| Circulatory arrest time (minutes) | 41 (32–113) | 32 (0–58) | 0.002 ⁎ |
| Left atrioventricular valve/right atrioventricular valve annulus | 0.35 ± 0.12 | 0.53 ± 0.09 | <0.001 ⁎ |
| Left atrioventricular valve/right atrioventricular valve index | 0.32 ± 0.16 | 0.64 ± 0.16 | <0.001 ⁎ |
| Z-score aortic annulus | −2.24 ± 1.32 | −1.20 ± 0.84 | 0.003 ⁎ |
| Z-score transverse arch | −3.10 ± 0.28 | −2.35 ± 0.60 | <0.001 ⁎ |
| Z-score isthmus | −3.60 ± 0.59 | −2.80 ± 0.97 | 0.002 ⁎ |
| Left ventricular volume/m 2 (cm 3 /m 2 ) | 2.60 (0.14–14.6) | 13.4 (4.0–28.1) | <0.001 ⁎ |
| Left ventricular length/right ventricular length | 0.82 (0.15–0.96) | 0.98 (0.67–1.11) | <0.001 ⁎ |
| Left/right primary inflow | 0.33 ± 0.12 | 0.53 ± 0.11 | <0.001 ⁎ |
| Left/right secondary inflow | 0.31 ± 0.15 | 0.51 ± 0.14 | 0.001 ⁎ |
| Left ventricular inflow index | 0.72 ± 0.20 | 0.71 ± 0.14 | 0.72 |
| Retrograde transverse arch flow with duct | 14/20 (70%) | 3/11 (27%) | 0.03 ⁎ |
| Left atrioventricular valve morphologic abnormal | 11/23 (48%) | 5/22 (23%) | 0.12 |
| Any right to left flow at duct | 20/20 (100%) | 8/11 (72%) | 0.052 |
| Average ventricular septal defect size (cm) | 0.46 ± 0.14 | 0.5 ± 0.20 | 0.54 |
| Moderate or greater atrioventricular valve regurgitation | 4/23 (18%) | 7/22 (32%) | 0.31 |
| Any genetic syndrome | 2/23 (8.7%) | 6/22 (27.3%) | 0.13 |
| Need for coarctation repair | 23/23 (100%) | 7/22 (32%) | <0.001 ⁎ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


