Patent foramen ovale (PFO) is associated with cryptogenic stroke, migraine headache, decompression sickness, and platypnea-orthodeoxia syndrome. Patients undergoing transesophageal echocardiography are often hypovolemic from preprocedural fasting and might not demonstrate right to left shunting owing to insufficient right atrial pressure generation, despite provocative maneuvers. We hypothesized that volume replenishment with saline loading could potentially unmask a PFO by favorably modulating the interatrial pressure gradient. Our study sought to examine the role of pre- or intraprocedural intravenous fluid replenishment on PFO detection during transesophageal echocardiography. A total of 103 patients were enrolled. An initial series of bubble injections was performed unprovoked and then with provocative maneuvers such as the Valsalva maneuver and coughing. The patients were then given a rapid 500 ml saline bolus, and the same sequence of bubble injections was repeated. The presence, type, and magnitude of the right to left shunts were noted before and after the saline bolus. The detection rate of PFO increased from 10.6% to 26.2% after saline loading without any provocative maneuvers. When combined with provocative maneuvers (Valsalva or cough), saline loading improved the detection rate from 17.4% to 32.0%. Overall, from amongst the 103 enrolled patients, saline bolusing resulted in a de novo diagnosis of PFO in 15 patients, atrial septal aneurysm in 15, PFO coexisting with an atrial septal aneurysm in 10, and pulmonary arteriovenous fistula in 5 patients. In conclusion, saline infusion in appropriately selected patients during transesophageal echocardiography significantly enhances the detection of PFOs and pulmonary arteriovenous fistulas.
In an attempt to examine the effect of pre- or intraprocedural intravenous fluid replenishment (saline bolus) on patent foramen ovale (PFO) detection rates, we prospectively enrolled 103 consecutive patients referred for transesophageal echocardiography (TEE) at a tertiary care medical center. All patients underwent a series of agitated saline bubble injections (with and without provocative maneuvers) before and after an intravenous saline bolus.
Methods
The present prospective experimental study was conducted from May 2008 to September 2009. The institutional review board reviewed and approved the protocol, in compliance with HIPAA for investigator participation in the study before data use.
A total of 211 adult patients scheduled for TEE for a variety of indications, as a part of their routine clinical care, were screened for inclusion. Of these, 103 patients fulfilled the inclusion criteria and were considered in the final analysis.
The exclusion criteria (n = 108) included a left ventricular ejection fraction <30% (n = 32), New York Heart Association class III-IV (n = 12), mechanically ventilated patients (n = 20), end-stage renal disease (n = 36), known intracardiac shunts (n = 4), inadequate opacification of right atrium with contrast (n = 2), and miscellaneous reasons (n = 2).
A detailed cross-sectional survey of paper and electronic medical records was undertaken for demographic and clinical data. Upon obtaining informed consent from the patients, local anesthesia and conscious sedation were administered according to our laboratory routine. After intubation in standard manner, a minimum of 3 contrast injections (presaline loading) were performed as detailed in the protocol flow diagram ( Figure 1 ). The patients’ vital signs were monitored according to the laboratory protocol throughout the procedure and during recovery.
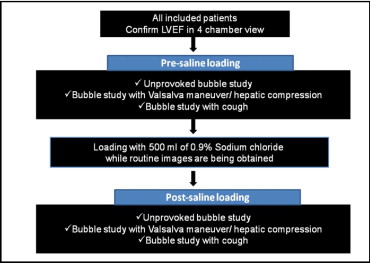
All transesophageal echocardiographic images were acquired using a commercial ultrasound machine (Vivid 7, GE, Vingmed, Horten, Norway) with a GE 6 T, multiplane transesophageal echocardiographic probe (2.9 to 7.0 MHz) and images were digitally stored for off-line analysis. All machine settings were optimized before image acquisition, and no further adjustments made during the course of the study. Digitized video loops of sufficient length (≥15 beats) were acquired using standard views and analyzed off-line by 2 independent reviewers. The 90° mid-esophageal bicaval view was used for all injections, with additional views to profile the pulmonary veins as appropriate, in patients with suspected pulmonary arteriovenous fistulas (PAVFs). Conflicting results were adjudicated by consensus. All qualifying patients underwent a rapid fluid bolus of 500 ml of 0.9% normal saline within 25 to 30 minutes, delivered intravenously by way of a forearm vein, with the assistance of a pressure bag (inflated to 300 mm Hg). On completion of the saline infusion, a repeat series of bubble injections was performed in identical fashion, and digitized loops were similarly stored for comparative analysis. Each set included a minimum of 3 injections (1 injection without provocation and 2 with provocative maneuvers, as outlined in Figure 1 ).
For generation of saline contrast, we used two 10-ml syringes, attached to a 3-way stopcock; 1 of the syringes was filled with 9.0 ml saline (0.9% NaCl) and 1 with 1.0 ml of air. After attaching the setup to the intravenous access line with sterile precautions, the saline was vigorously injected into the empty syringe back and forth 3 to 5 times to generate a uniform, opalescent suspension of microbubbles, following which the contrast admixture was rapidly injected into the forearm vein (using a 21-gauge intravenous catheter).
A PFO was diagnosed if passage of saline bubble contrast was observed in the left atrium within 3 to 5 cardiac cycles of complete opacification of the right atrium, if left atrial contrast was observed coincident with a provocative maneuver, or if bubble transit/exit was observed by way of the PFO tunnel, during the frame-by-frame, off-line analysis.
Late appearance of bubble contrast in the left atrium (>6 to 8 beats after right atrial opacification), or after contrast was no longer visualized in the right atrium was deemed secondary to a PAVF. Attempts were made to independently confirm bubble contrast emerging from the pulmonary vein ostia in the subset of patients with suspected transpulmonary shunting.
De novo PFO or PAVF was diagnosed when a PFO or PAVF not evident in the baseline set of contrast injections was unmasked after fluid replenishment. An atrial septal aneurysm (ASA) was diagnosed if the maximum excursion of the mobile interatrial septum measured ≥1.0 cm.
The shunt size or degree of right-to-left shunting (RLS) was further semiquantitatively evaluated and stratified as 0, no shunt (<3 microbubbles); 1, small shunt (3 to 9 microbubbles); 2, moderate shunt (10 to 30 microbubbles); 3, large shunt (>30 microbubbles). RLS was assessed in a similar manner for both sets of bubble injections. Careful note was again made of de novo diagnosed PFOs (detected after saline infusion), and the magnitude of shunting was compared before and after saline loading. Echocardiographic images of patients from the study cohort were assigned to 2 certified echocardiographers (A.K. and M.S.), who independently interpreted the presence or absence of PFO in a blinded manner. An interrater reliability analysis was performed to determine the consistency among observers using the κ statistic.
The mean and SDs of measurement differences for continuous variables (before and after saline loading) were calculated, and a paired t test was used to test the significance of these differences. Similarly, between group comparisons for categorical variables were made using the chi-square test.
The detection rate of PFO on TEE before and after saline loading was calculated using the number of positive bubble studies for each modality divided by the total number of patients in the study cohort (n = 103).
The following estimates were made: (1) detection rate of PFO on TEE before saline loading was estimated as the number of positive studies before saline loading divided by 103 patients; and (2) the detection rate of PFO on TEE after saline loading was estimated as the number of positive studies after saline loading divided by 103 patients. The detection rates of PFO on TEE with and without provocative maneuvers, before and after the saline bolus, were also calculated in a similar manner. A 2-tailed p <0.05 was considered statistically significant for all analyses. All statistical analyses were performed using the Statistical Package for Social Sciences, version 15.0 (SPSS, Chicago, Illinois).
Results
The study cohort consisted of 103 participants (58.2% women), with a mean age of 51.2 ± 15 years. The indications for TEE included evaluations for PFO (43%), endocarditis (32%), valvular heart disease (15%), thrombus (9%), and a right atrial mass (1%).
A total of 33 patients were detected with a PFO in this study cohort. Bubble injections performed before the saline bolus without and with provocation identified 11 and 18 cases of PFO, respectively. The overall detection rate of TEE to identify PFO improved significantly after saline infusion ( Figure 2 and Videos 1 and 2 ). Saline bolusing in the absence of provocation identified a total of 27 PFOs (detection rate 26.2%); with provocation, this improved further to 33 cases (detection rate 32%). In all, 15 de novo PFOs were identified using a 500-ml bolus in the enrolled population. Thus, compared to the conventional method (i.e., with provocation but without a saline bolus), a saline bolus infusion with provocation improved the detection rate of PFO on TEE by 84% (from 17.4% to 32%).
Furthermore, in the small and moderate shunt category, the shunt size was exaggerated after the saline bolus in a significant proportion of patients ( Figure 3 ).
A similar effect of an improved detection rate was observed when the maximum atrial septal excursion was measured during TEE, before and after saline loading. Before the saline infusion, 11 patients were found to have an ASA (maximum atrial septal excursion of ≥1.0 cm); after saline bolus, de novo ASA was demonstrated in an additional 15 patients, and a PFO was evident in 10 of these patients.
Before the saline bolus, 5 of the 103 patients had evidence of left to right shunting at the atrial level, as demonstrated by color flow Doppler. In the initial set of bubbles studies before saline infusion, except for 1 patient, none had evidence of RLS. After the saline infusion, RLS was detected in 4 of the 5 patients. In the patient with RLS at baseline, the magnitude of right to left shunting increased after saline infusion.
Before the saline bolus, an excellent agreement was observed with a κ of 0.88 (p <0.001) and 0.77 (p <0.001) for unprovoked and provoked bubble studies, respectively. On the postsaline bolus echocardiographic images, an agreement for the presence or absence of PFO was substantial with a κ of 0.66 (p <0.001) and 0.76 (p <0.001) for unprovoked and provoked bubble study images, respectively. Finally, for all the study images analyzed, the overall level of agreement for the presence of PFO between the 2 observers was very good, with a κ of 0.80 (range 0.71 to 0.89, p <0.001).
In clinical terms, of the 103 enrolled patients, a single 500-ml saline bolus enabled us to establish a de novo diagnosis of PFO in 15 patients, ASA in 15, PFO coexisting with an ASA in 10, and PAVF in 5 patients ( Figure 4 ). Of the 44 patients undergoing TEE specifically for evaluation of an intracardiac shunt, a de novo diagnosis of PFO was made in 8, ASA in 5, and PFO coexisting with ASA in 3 patients.




