Intra-aortic balloon pump (IABP) counterpulsation is the most widely used mechanical circulatory support device because of its ease of use, low complication rate, and fast manner of insertion. Its benefit is still subject of debate, and a considerable gap exists between guidelines and clinical practice. Retrospective nonrandomized studies and animal experiments show benefits of IABP therapy. However, recent large randomized trials do not show benefit of IABP therapy, which has led to a downgrading in the guidelines. In our view, this dichotomy between trials and practice might be the result of insufficient understanding of the prerequisites needed for effective IABP therapy, that is, exhausted autoregulation, and of not including the right patient population in trials. The population included in recent large randomized trials has been heterogeneous, also including patients in whom benefit of IABP could not be expected. The clinical condition in which most benefit is expected, that is persistent ischemia in acute ST-elevation myocardial infarction, is discussed in this review. In conclusion, this review aims to explain the physiological principles needed for effective IABP therapy, to reflect critically on the large randomized trials, and to solve some of the controversies in this field.
Appropriate use of intra-aortic balloon pump (IABP) counterpulsation has been subject to heavy debate over the past years. Use of IABP is generally confined to 3 groups of patients, that is, high-risk percutaneous coronary intervention (PCI), acute myocardial infarction, and cardiogenic shock. There have been large randomized trials for all 3 indications, which will be discussed in the following sections. However, before analyzing these trials in detail, it is mandatory to better understand the presumed physiological principles of IABP counterpulsation and the prerequisites needed for adequate effect (or absence of effect) of IABP.
Physiological Principles and the Role of Coronary Autoregulation
The IABP, positioned in the descending thoracic aorta through the femoral artery, inflates and deflates in synchrony with the cardiac cycle. By inflating in the diastolic phase of the cardiac cycle, the diastolic aortic pressure is augmented, resulting in higher coronary perfusion pressure ( Figure 1 ). This could theoretically lead to improved coronary blood flow and thereby increase of myocardial oxygen supply.
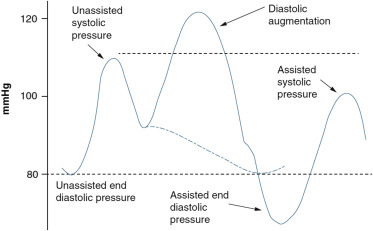
Early in systole, the balloon rapidly deflates, reducing the afterload of the left ventricle ( Figure 1 ). In turn, this is believed to decrease myocardial workload and oxygen demand. However, the supposed direct mechanical effect of counterpulsation on coronary blood flow can be easily undone by the reactive vasoconstriction of the coronary and myocardial bed, known as coronary autoregulation. Under normal physiological circumstances, sphincters at the entrance of coronary arterioles constrict or dilate in response to coronary perfusion pressure, thereby guaranteeing constant myocardial blood flow over a wide range of aortic pressures (60 to 140 mm Hg). Therefore, in physiologic conditions with intact coronary autoregulation, myocardial blood flow is not dependent on perfusion pressure, and it is illusionary to expect increased coronary blood flow because of higher perfusion pressure by IABP counterpulsation. Thus, improved coronary blood flow by IABP can only be expected in situations with exhausted autoregulation.
Recently, De Silva et al performed intracoronary flow and pressure measurements during IABP counterpulsation with “switched on” and “switched off” coronary autoregulation using intravenous adenosine infusion for minimizing coronary vascular resistance. The investigators showed that the effect of IABP on coronary flow is directly dependent on coronary autoregulation. With intact autoregulation, balloon pump augmentation did lead to an increase in coronary pressure and also to a reactive increase in microvascular resistance, and as a result unchanged coronary blood flow. In contrast, with “switched off” autoregulation, the balloon pump augmentation led to an increase in distal coronary pressure and coronary blood flow, whereas the microvascular resistance remained unchanged. Also in recent studies performed in the isolated beating pig heart, it was documented that with exhausted autoregulation, a linear relation between diastolic aortic pressure and coronary blood flow is present and that IABP increases coronary blood flow by up to 50% in the presence of pump failure because of ongoing myocardial ischemia.
In short, only when autoregulation is exhausted, coronary blood flow is directly dependent on perfusion pressure, and effects of IABP on coronary blood flow can be expected. This is the case in the following clinical situations: (1) in the perfusion territory of a critical, subtotal stenosis; (2) in ischemic myocardium, including “stunning” after myocardial infarction or during weaning from extracorporeal circulation; and (3) in patients with low mean aortic pressures outside the autoregulatory range (<60 mm Hg).
The importance of autoregulation when assessing the effect of IABP was neither fully understood nor investigated until recently and is not reflected in the inclusion criteria in the large randomized clinical trials, including acute myocardial infarction and cardiogenic shock.
High-Risk PCI
IABP is often used as circulatory support system to prevent major complications during high-risk PCI. Patients with severe left ventricular dysfunction undergoing PCI are at higher risk of morbidity and mortality. This risk increases when the target lesion is supplying a substantial proportion of the myocardium. By inducing ischemia during PCI, left ventricular function will further deteriorate, and patients are at risk of entering a downward spiral of myocardial ischemia, hemodynamic compromise, cardiogenic shock, and ultimately death. Especially if a subtotal stenosis is present in a large contralateral coronary artery, secondary ischemia of the contralateral myocardial territory due to compensatory hyperkinesia might induce a downward spiral with deleterious outcome.
Until 2010, evidence supporting use of IABP in such patients consisted of several retrospective observational and small randomized studies. The Balloon Pump–Assisted Coronary Intervention Study (BCIS-1) randomized 301 patients scheduled to undergo high-risk single-vessel or multivessel PCI to either IABP insertion before PCI or no planned IABP insertion. On the basis of the predicted and actual event rates, the study was adequately powered. Outcome at hospital discharge was similar between the 2 groups, without significant differences in myocardial infarction, death, cerebrovascular accident, or further revascularization. In the control group, 12% of the patients needed rescue IABP insertion because of intraprocedural complications, mainly hypotension. Six-month mortality was 4.6% in the IABP group and 7.4% in the control group (p = 0.32). Although not statistically significant at first, this relative risk reduction of 38% remained constant over time, resulting in a significant benefit in favor of IABP over long-term follow-up, with a hazard ratio of 0.66 (95% confidence interval 0.44 to 0.98; p = 0.039). This consistent relative risk reduction by 1/3 is of huge impact in a patient population in which 1 out of every 3 patients died within the long-term follow-up period ( Figure 2 ). The constant hazard ratio during follow-up implies that this is attributable to an early treatment effect, that is, IABP insertion before PCI. There were no other detectable procedural differences between the 2 groups in terms of number of vessels treated, success rate, and proportion of left main or proximal left anterior descending coronary arteries stented.
Explanation of this difference in outcome could be the reduction of ischemia by IABP during high-risk PCI and the prevention of intraprocedural complications. Effects of IABP on coronary blood flow is not undone by coronary autoregulation in these patients because autoregulation is completely exhausted in the coronary tree distal to a significant stenosis and in the presence of ischemic myocardium during and shortly after balloon inflation. Even small periprocedural myocardial infarctions, only measured by elevated troponin levels, have shown to affect outcome in cardiac patients. In patients with severe ischemic cardiomyopathy as included in BCIS-1, this effect might be more pronounced, causing this significant difference in long-term outcome. It is well conceivable that better protection of the myocardium during the procedure itself will result in a decrease in mortality in the long term, explaining the late results of BCIS-1.
High-Risk PCI
IABP is often used as circulatory support system to prevent major complications during high-risk PCI. Patients with severe left ventricular dysfunction undergoing PCI are at higher risk of morbidity and mortality. This risk increases when the target lesion is supplying a substantial proportion of the myocardium. By inducing ischemia during PCI, left ventricular function will further deteriorate, and patients are at risk of entering a downward spiral of myocardial ischemia, hemodynamic compromise, cardiogenic shock, and ultimately death. Especially if a subtotal stenosis is present in a large contralateral coronary artery, secondary ischemia of the contralateral myocardial territory due to compensatory hyperkinesia might induce a downward spiral with deleterious outcome.
Until 2010, evidence supporting use of IABP in such patients consisted of several retrospective observational and small randomized studies. The Balloon Pump–Assisted Coronary Intervention Study (BCIS-1) randomized 301 patients scheduled to undergo high-risk single-vessel or multivessel PCI to either IABP insertion before PCI or no planned IABP insertion. On the basis of the predicted and actual event rates, the study was adequately powered. Outcome at hospital discharge was similar between the 2 groups, without significant differences in myocardial infarction, death, cerebrovascular accident, or further revascularization. In the control group, 12% of the patients needed rescue IABP insertion because of intraprocedural complications, mainly hypotension. Six-month mortality was 4.6% in the IABP group and 7.4% in the control group (p = 0.32). Although not statistically significant at first, this relative risk reduction of 38% remained constant over time, resulting in a significant benefit in favor of IABP over long-term follow-up, with a hazard ratio of 0.66 (95% confidence interval 0.44 to 0.98; p = 0.039). This consistent relative risk reduction by 1/3 is of huge impact in a patient population in which 1 out of every 3 patients died within the long-term follow-up period ( Figure 2 ). The constant hazard ratio during follow-up implies that this is attributable to an early treatment effect, that is, IABP insertion before PCI. There were no other detectable procedural differences between the 2 groups in terms of number of vessels treated, success rate, and proportion of left main or proximal left anterior descending coronary arteries stented.
Explanation of this difference in outcome could be the reduction of ischemia by IABP during high-risk PCI and the prevention of intraprocedural complications. Effects of IABP on coronary blood flow is not undone by coronary autoregulation in these patients because autoregulation is completely exhausted in the coronary tree distal to a significant stenosis and in the presence of ischemic myocardium during and shortly after balloon inflation. Even small periprocedural myocardial infarctions, only measured by elevated troponin levels, have shown to affect outcome in cardiac patients. In patients with severe ischemic cardiomyopathy as included in BCIS-1, this effect might be more pronounced, causing this significant difference in long-term outcome. It is well conceivable that better protection of the myocardium during the procedure itself will result in a decrease in mortality in the long term, explaining the late results of BCIS-1.
Acute Myocardial Infarction
Primary PCI in patients presenting with ST-segment elevation myocardial infarction (STEMI) has resulted in an impressive improvement in outcome. During the last decade, however, despite optimizing time intervals, outcome has not further improved. This may be ascribed to “reperfusion injury,” “no-reflow,” or “persistent ischemia,” which occurs in up to 30% of the patients and is caused by a variety of factors including microembolization of atherothrombotic debris, vasospasm, and external compression of the capillaries due to intramyocardial edema, hemorrhage, and other factors, suppressing adequate myocardial perfusion after successful epicardial stenting. In such patients, this “ongoing” or “persistent” ischemia leads to reduced salvage of myocardium, even if the occluded epicardial coronary artery has been opened successfully by primary PCI.
Animal experiments have shown that IABP is effective in reducing no-reflow, improves myocardial salvage, and decreases infarct size. A clinical registry in nearly 1,500 consecutive patients showed prophylactic insertion of IABP to be associated with fewer events in all high-risk patients presenting with acute myocardial infarction.
The Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction (CRISP AMI) trial sought to determine if routine IABP therapy before primary PCI in patients with anterior STEMI without shock reduces infarct size as assessed on cardiac magnetic resonance imaging (CMR) and randomized 337 patients. The study was designed and powered to detect a 25% reduction in infarct size in the IABP group. Final infarct size as assessed by CMR after 3 to 5 days showed no difference and a trend toward larger infarctions in the IABP group (42.1% vs 37.5%, p = 0.06). All-cause mortality at 6 months occurred less frequent in the IABP group, although not statistically significant (2% vs 5%, p = 0.12). The exploratory composite end point of death, shock, or new or worsening heart failure did reach statistical significance in favor of the IABP group (5% vs 12%, p = 0.03). So, in CRISP AMI, the primary end point was not achieved, and some confusion originated with respect to other end points.
However, 40% of the population in CRISP AMI had summed ST-elevation <6 mm, representing a cohort of patients with relatively small infarctions with a good prognosis anyway, whether treated with IABP or any other additional therapy. It is unlikely that in these patients, any of the predefined end points like death or congestive heart failure would be reached anyway, irrespective if and how they were treated. This is corroborated by the systolic and diastolic blood pressure and heart rate of the study population, not suggesting a very sick trial population. Inclusion of these patients most likely diluted a possible effect of IABP insertion by underpowering the study. A recent substudy of CRISP AMI in patients with large myocardial infarction (summed ST-deviation ≥15 mm) and persistent ischemia (ST-resolution after PCI <50%) showed a significant reduction in mortality despite a much smaller patient population ( Figure 3 ).




