Whether hyponatremia (sodium <135 mEq/L) in the acute phase of ST-segment elevation myocardial infarction is just a marker of “more ill” patients or decreased sodium concentration is able to exert a direct adverse effect on the cardiovascular system is still unknown. The aim of this study was to assess the prognostic impact, in the short and long terms, of admission hyponatremia in 1,231 consecutive patients with ST-segment elevation myocardial infarctions all submitted to primary percutaneous coronary intervention. In this series, 286 patients (23.2%) had sodium values <135 mEq/L. Patients with hyponatremia were older (p = 0.018) and more frequently had diabetes (p = 0.040). Anterior myocardial infarction was more frequent in patients with hyponatremia, who showed a higher incidence of 3-vessel coronary artery disease and advanced Killip class. Higher mortality rates were observed in patients with hyponatremia during intensive cardiac care unit stay and at follow-up. On multivariate regression analysis, admission sodium concentration was not independently related to early death, nor did it show any relations with long-term mortality on Cox regression analysis. In conclusion, the main findings of the present investigation are as follows: (1) hyponatremia is a common finding, being associated mainly with older age, diabetes, and advanced Killip class; (2) patients with hyponatremia had higher rates of in-hospital and long-term mortality; and (3) hyponatremia, also when assessed by means of the propensity score model, was not independently associated with increased risk for death in the short and long terms. These data therefore strongly suggest that the presence of hyponatremia in the acute phase of ST-segment elevation myocardial infarction should be considered a marker of more ill patients.
Hyponatremia (sodium <135 mEq/L) in the acute phase of ST-segment elevation myocardial infarction (STEMI) has been identified as an independent predictor of short-term mortality, long-term mortality, and rehospitalization for heart failure. However, previous investigations were performed in the thrombolytic era or included series of heterogenous patients with STEMIs submitted either to thrombolysis or percutaneous coronary intervention. Whether hyponatremia is just a marker of “more ill” patients or decreased sodium concentration is able to exert a direct adverse effect on the cardiovascular system is still unknown. We assessed the prognostic impact, in the short and long terms, of admission hyponatremia in 1,231 consecutive patients with STEMIs, all submitted to primary percutaneous coronary intervention (PCI) and admitted to our intensive cardiac care unit (ICCU).
Methods
From April 1, 2004, to December 31, 2010, 1,231 consecutive patients with STEMIs (within 12 hours of symptom onset) were admitted to our ICCU, which is located at a tertiary center (at which primary PCI can be performed 24 hours a day, 7 days a week). In our hospital, in Florence, Italy, the reperfusion strategy for patients with STEMIs is represented by primary PCI. Patients with STEMIs are first evaluated by the medical emergency system staff in the prehospital setting and then directly admitted to the catheterization laboratory or transferred there after rapid stabilization in the first aid department. After primary PCI, they are admitted to our ICCU.
A successful procedure was defined as an infarct-related artery stenosis <20% associated with Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow. Failed PCI was defined as resulting in TIMI grade 0 to 2 flow, regardless residual stenosis.
The diagnosis of STEMI was based on the criteria of the American College of Cardiology and American Heart Association.
On ICCU admission, after PCI, in a fasting blood sample, the following parameters were measured: sodium (milliequivalents per liter), glucose (grams per liter), glycosylated hemoglobin (percentage), troponin I (nanograms/milliliter), uric acid (milligrams per deciliter), N-terminal pro–brain natriuretic peptide (picograms per milliliter), erythrocyte sedimentation rate, leukocyte count (thousands per microliter), fibrinogen (milligrams per deciliter) and high-sensitivity C-reactive protein positivity. Creatinine (milligrams per deciliter) was also measured to calculate the glomerular filtration rate (milliliters per minute per 1.73 square meters), on admission and at discharge. Glucose, troponin I, and creatinine were measured 3 times a day, and peak values were considered. Acute insulin resistance was assessed using homeostasis model assessment, as previously described. Mild hyponatremia was defined as sodium <135 mEq/L.
Transthoracic 2-dimensional echocardiography was performed on ICCU admission to measure the left ventricular ejection fraction (LVEF).
Ventilatory support (invasive and noninvasive ventilation), ultrafiltration (continuous venous-venous ultrafiltration, continuous venovenous hemodiafiltration), and intra-aortic balloon pump implantation were used when needed.
The primary end point was in-ICCU death and all-cause death at follow-up.
The study protocol was in accordance with the Declaration of Helsinki and approved by the local ethics committee. Written informed consent was obtained from all patients before enrollment.
Data were analyzed using SPSS version 17.0 (SPSS, Inc., Chicago, Illinois). A p value <0.05 was considered statistically significant. Discrete variables are reported as frequencies and percentages, and between-group comparisons were made using chi-square or Fisher’s exact tests, when the expected value in almost 1 cell was <5. Continuous variables are reported as mean ± SD or as median (interquartile range) according to the shape of their distribution, assessed using 1-sample Kolmogorov-Smirnov tests; comparisons were performed using Student’s t tests or Mann-Whitney U tests. A multivariate logistic regression model was constructed to identify adjusted predictors of in-ICCU death; candidate variables were carefully chosen among those significantly different on univariate analysis and/or clinically relevant, to avoid model overfitting. Model calibration was assessed using the Hosmer-Lemeshow test.
Because several baseline characteristics of patients with hyponatremia significantly differed from those with normal sodium levels, a propensity analysis was carried out using a nonparsimonious logistic regression to determine the probability of a patient to have serum sodium <135 mEq/L. The variables included in the propensity score model were age; body mass index; glycemia; estimated glomerular filtration rate (eGFR); leukocyte count; fibrinogen; previous PCI; history of diabetes; coronary disease severity; myocardial infarction location; Killip classification; treatment with diuretics, nitrates, and inotropes; mechanical ventilation; continuous renal replacement therapy; and intra-aortic balloon pump implantation. The procedure selected 260 patients with hyponatremia to be compared to 260 with normal sodium levels; all baseline characteristics (except myocardial infarction location) did not differ between the 2 subgroups.
After the assessment of risk proportionality, a multivariate Cox regression analysis was conducted to identify predictors of long-term death.
Results
In our series, 286 of 1,231 patients (23.2%) showed sodium values <135 mEq/L. Patients with hyponatremia patients were older (p = 0.018) and more frequently had diabetes (p = 0.040). Anterior wall myocardial infarction was more frequent in patients with hyponatremia, who showed a higher incidence of 3-vessel coronary artery disease and advanced Killip class. Higher mortality rates were observed in patients with hyponatremic during the ICCU stay and at follow-up ( Table 1 ).
| Variable | Serum Sodium (mEq/L) | p Value | |
|---|---|---|---|
| <135 | ≥135 | ||
| (n = 286) | (n = 945) | ||
| Age (years) | 68.4 ± 11.9 | 66.3 ± 12.9 | 0.018 |
| Men/women | 198 (69.2%)/88 (30.8%) | 699 (74.0%)/246 (26.0%) | 0.114 |
| Body mass index (kg/m 2 ) | 25.7 ± 3.3 | 26.4 ± 3.7 | 0.007 |
| Diabetes mellitus | 85 (29.7%) | 224 (23.7%) | 0.040 |
| Smoking | 168 (58.7%) | 598 (63.3%) | 0.165 |
| Chronic obstructive pulmonary disease | 27 (9.4%) | 77 (8.1%) | 0.491 |
| Previous PCI | 48 (16.8%) | 120 (12.7%) | 0.078 |
| Previous myocardial infarction | 48 (16.8%) | 126 (13.3%) | 0.142 |
| Hypertension | 157 (54.9%) | 501 (53.0%) | 0.577 |
| Symptoms-to-balloon time (minutes) | 240 (180–350) | 230 (160–300) | 0.078 |
| Acute myocardial infarction location | 0.005 | ||
| Anterior | 175 (61.2%) | 480 (50.8%) | |
| Inferior | 89 (31.1%) | 393 (41.6%) | |
| Other | 22 (7.7%) | 72 (7.6%) | |
| Number of coronary arteries narrowed | 0.048 | ||
| 1 | 96 (33.6%) | 389 (41.2%) | |
| 2 | 101 (35.3%) | 315 (33.3%) | |
| 3 | 89 (31.1%) | 241 (25.5%) | |
| Left main | 14 (4.9%) | 43 (4.6%) | 0.808 |
| Coronary artery bypass grafting | 6 (2.1%) | 24 (2.5%) | 0.671 |
| PCI failure | 23 (8.1%) | 54 (5.8%) | 0.166 |
| Killip class | 0.012 | ||
| I or II | 243 (85.0%) | 853 (90.3%) | |
| III or IV | 43 (15.0%) | 92 (9.7%) | |
| Length of stay (hours) | 72 (48–96) | 61 (48–79) | 0.051 |
| In-ICCU death | 22 (7.7%) | 36 (3.8%) | 0.007 |
| Follow-up death (n = 530) | 52/237 (21.9%) | 111/820 (13.5%) | 0.002 |
As listed in Table 2 , patients with hyponatremia had higher values of admission and peak glycemia (p <0.001 and p <0.001, respectively), homeostasis model assessment of insulin resistance positivity (p = 0.038), and lower values of admission, nadir, and discharge eGFR (p <0.001 for all). Higher values of N-terminal pro–brain natriuretic peptide were observed in patients with hyponatremia (p = 0.002), as well as higher levels of erythrocyte sedimentation rate (p = 0.003), leukocyte count (p = 0.003), and fibrinogen (p = 0.011).
| Variable | All Patients (n = 1,231) | Serum Sodium (mEq/L) | p Value | |
|---|---|---|---|---|
| <135 (n = 286) | ≥135 (n = 945) | |||
| Admission glucose (mg/L) | 133 (112–169) | 140 (116–192) | 130 (110–163) | <0.001 |
| Peak glycemia (mg/L) | 152 (127–197) | 165 (134–232) | 150 (125–189) | <0.001 |
| Insulin (mU/L) | 9.6 (5.5–17.7) | 9.2 (5.0–20.3) | 9.8 (5.7–17.3) | 0.664 |
| Homeostasis model assessment of insulin resistance positivity | 116/720 (16.1%) | 35/164 (21.3%) | 81/556 (14.6%) | 0.038 |
| Glycosylated hemoglobin (%) | 5.9 (5.6–6.4) | 6.0 (5.7–7.0) | 5.9 (5.6–6.4) | 0.019 |
| eGFR (ml/min/1.73 m 2 ) | ||||
| Admission | 83.0 ± 30.2 | 77.4 ± 31.2 | 84.6 ± 29.7 | <0.001 |
| Nadir | 69.9 ± 25.9 | 62.6 ± 27.6 | 72.2 ± 25.0 | <0.001 |
| Discharge | 79.9 ± 28.7 | 73.8 ± 31.6 | 81.8 ± 27.5 | <0.001 |
| Microalbuminuria (mg/dl) | 17.0 (6.4–55.0) | 16.0 (6.4–73.2) | 17.2 (6.4–53.8) | 0.407 |
| Peak troponin I (ng/ml) | 82.7 (36.9–176.2) | 95.5 (38.6–204.8) | 79.2 (36.1–165.9) | 0.116 |
| N-terminal pro–brain natriuretic peptide (pg/ml) | 1,306 (468–3,257) | 1,722 (568–4,707) | 1,140 (459–2,758) | 0.002 |
| Uric acid (mg/dl) | 5.7 ± 1.8 | 5.6 ± 1.8 | 5.8 ± 1.8 | 0.354 |
| Erythrocyte sedimentation rate (mm/hour) | 24 (12–41) | 27 (14–49) | 23 (12–38) | 0.003 |
| Leukocyte count (×10 3 /μl) | 10.7 (8.8–13.7) | 11.6 (9.4–14.6) | 10.7 (8.7–13.4) | 0.003 |
| High-sensitivity C-reactive protein positivity | 456/913 (49.9%) | 119/227 (52.4%) | 337/686 (49.1%) | 0.389 |
| Fibrinogen (mg/dl) | 398 (335–479) | 417 (337–523) | 391 (334–468) | 0.011 |
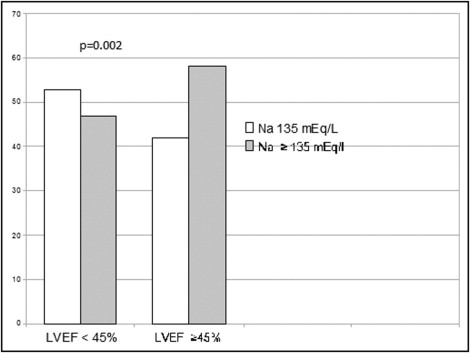
In patients with admission LVEFs <45%, the incidence of hyponatremia was higher with respect to that observed in patients with admission LVEFs >45% (p = 0.002; Figure 1 ).
As listed in Table 3 , higher use of ventilatory support, intra-aortic balloon pump implantation, and ultrafiltration was observed in patients with hyponatremia.
| Variable | All Patients (n = 1,231) | Serum Sodium (mEq/L) | p Value | |
|---|---|---|---|---|
| <135 (n = 286) | ≥135 (n = 945) | |||
| Mechanical ventilation | 91/1,215 (7.5%) | 32/283 (11.3%) | 59/932 (6.3%) | 0.005 |
| Noninvasive ventilation | 70/1,192 (5.9%) | 25/278 (9.0%) | 45/914 (4.9%) | 0.012 |
| Continuous venovenous hemodiafiltration | 47/283 (3.9%) | 22/280 (7.9%) | 25/933 (2.7%) | <0.001 |
| Intra-aortic balloon pump | 274/1,213 (22.6%) | 83/282 (29.4%) | 191/931 (20.5%) | 0.002 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


