The objective of the present study was to test whether a perioperative course of colchicine, in patients who underwent standard coronary artery bypass grafting, would result in reduced postoperative increase of myocardial injury biomarker levels. Patients were prospectively randomized to colchicine or placebo starting 48 hours before scheduled coronary artery bypass grafting and for 8 days thereafter (0.5 mg twice daily). The primary outcome parameter was maximal high-sensitivity troponin T (hsTnT) concentration within 48 hours after surgery. Secondary outcome measures were maximal creatine kinase-myocardial brain fraction (CK-MB) levels and area under the curve (AUC) of hsTnT and CK-MB concentrations; 59 patients were included. Maximal hsTnT was 616 pg/ml (396 to 986) in the colchicine group versus 1,613 pg/ml (732 to 2,587) in controls (p = 0.002). Maximal CK-MB was 44.6 ng/ml (36.6 to 68.8) and 93.0 ng/ml (48.0 to 182.3), respectively (p = 0.002). The median AUC for hsTnT was 40,755 pg h/ml (20,868 to 79,176) in controls versus 20,363 pg h/ml (13,891 to 31,661) in the colchicine group (p = 0.002). AUCs for CK-MB were 2,552 ng h/ml (1,564 to 4,791) in controls and 1,586 ng h/ml (1,159 to 2,073) in the colchicine group (p = 0.003). The main complaints associated with colchicine were, as expected, gastrointestinal, with 5 patients (16.7%) in the colchicine group reporting diarrhea versus 1 control (3.4%) (p = 0.195). In conclusion, a short perioperative course of colchicine was effective in attenuating postoperative increases of hsTnT and CK-MB compared with placebo. This finding, which needs confirmation in a larger clinical trial powered to assess clinical endpoints, suggests a potential role for this agent in reducing cardiac surgery–related myocardial damage.
Inflammatory responses and myocardial damage inflicted by cardiothoracic surgery have been shown to be a major contributor to postoperative morbidity and mortality. The objective of the present clinical trial ( ClinicalTrials.gov Identifier: NCT02122484 ) was to test whether a short perioperative course of colchicine, in patients who underwent standard coronary artery bypass grafting (CABG), would result in reduced postoperative increase of myocardial damage biomarker levels.
Methods
This was a prospective, double-blinded, placebo-controlled study. Patients who underwent elective CABG in a tertiary Athens metropolitan area General Hospital from December 2013 until June 2014 were included. Main exclusion criteria were age ≤18 or ≥80 years, concomitant valve surgery, CABG without cardiopulmonary bypass, acute coronary syndrome within the previous 4 weeks, inotropic or mechanical circulatory support before induction of anesthesia, any condition that could potentially increase preoperative troponin concentrations, active inflammatory disease, infectious disease or known malignancy, treatment with corticosteroids or anti-inflammatory agents, known hypersensitivity to colchicine, chronic treatment with colchicine, estimated glomerular filtration rate <35 ml/min/1.73 m 2 , and hepatic failure (Child-Pugh class B or C). The protocol was approved by the competent institutional review boards and was implemented in accordance with the provisions of the Declaration of Helsinki. All patients provided informed consent.
Patients were randomized (using a 1:1 allocation scheme based on a computer-generated randomization algorithm—an R language script was used for this purpose) to receive colchicine or placebo starting 48 hours before scheduled CABG and for 8 days after surgery. Colchicine was administered at a dose of 0.5 mg twice daily. Patients with <60 kg body weight received 0.5 mg once daily. Monitoring of adverse events focused on gastrointestinal manifestations, hepatotoxicity, myelotoxicity, and myotoxicity. Operators and personnel involved in patient follow-up and management were blinded as to patient allocation (patient treatment was identified by a serial number; assignment records were kept in a digital file which was unlocked after conclusion of last patient follow-up procedures and database locking).
All patients underwent CABG applying standard in-house protocols. After induction of anesthesia with intravenous propofol and fentanyl and neuromuscular blockade, the patient was ventilated under continuous propofol infusion and inspired isoflurane to maintain anesthesia. St. Thomas’ Hospital solution mixed with blood (3:1 ratio) was used for cardioplegia. Cardiopulmonary bypass and surgical techniques remained unchanged throughout the study period. A Sorin S5 system with a hollow fiber membrane oxygenator was used. Heparin was administered as needed to achieve activated clotting time ≥460 seconds. Systemic temperature was kept between 32°C and 34°C. All patients were transferred to the intensive care unit after surgery and then to the ward 48 to 72 hours later, if uncomplicated. Blood samples for high-sensitivity troponin T (hsTnT) (Elecsys Troponin T hs assay; Roche Diagnostics, Basel, Switzerland; lower detection limit 5 pg/ml) and creatine kinase-myocardial brain fraction (CK-MB) (Elecsys CK-MB assay; Roche Diagnostics; lower detection limit <0.1 ng/ml) measurement and other analyses (complete blood count, biochemistries, and so on) were obtained at baseline (on admission), intraoperatively (at the time of aortic clamping, designated “0 hours”) and then at 12, 24, 36, and 48 hours.
The primary outcome parameter was maximal hsTnT concentration within 48 hours after surgery. Secondary outcome measures were maximal CK-MB levels and area under the curve (AUC) of hsTnT and CK-MB concentrations (calculated using the Simpson’s summing rule).
Preliminary measurements in patients who underwent CABG in our institution showed a standard deviation of approximately 1,000 units in maximal postoperative hsTnT levels. It was calculated that to detect with 95% probability, at an alpha level (type I error probability) of 0.05, a difference between the 2 treatment groups equal to 1 SD, 27 subjects per group would have to be included. Analysis was performed on an intention-to-treat basis. Continuous variables were expressed as mean ± SD and compared using Gosset’s t test, if their distribution did not deviate significantly from the normal (tested with the Kolmogorov-Smirnov test). If significant deviation from the normal was found, continuous variables were expressed as median (first to third quartile) and were compared using nonparametric tests (Mann-Whitney U). Categorical variables were expressed as percentages and counts and compared using the chi-square test (or Fisher’s exact test, if the generated 2 × 2 contingency tables contained cells with expected counts equal or <5). The association of treatment with the primary outcome parameter (i.e., maximal hsTnT) was adjusted for predefined covariates and factors, namely hsTnT levels at 0 hours (to adjust for immediate preoperative values), time on extracorporeal circulation, and Euroscore II score (the selection of these potential explanatory variables was predefined and was made purely on grounds of clinical importance). Additional exploratory multivariable models were tested, including factors such as diabetes and history of ST-segment elevation myocardial infarction. Maximal hsTnT was entered (as the dependent variable) in all models after logarithmic transformation (to transform into a normal distribution). SPSS 17 software package (SPSS Inc., Chicago, Illinois) and R language were used for all analyses; p values <0.05 (2 sided) were considered as indicative of statistical significance.
Results
Of 75 screened patients, 60 consented and were entered in the study (30 per treatment arm). However, CABG was canceled in 1 patient of the placebo group (patient withdrew consent for surgery); as a result, 59 patients were finally included in the analytic cohort. No fatalities occurred during the procedure or the immediate postoperative period (i.e., all patients were discharged from hospital). Clinical and procedural information are summarized in Table 1 . Adherence was quite high, with 52 of 59 patients (88%) completing all 10 days of treatment; 4 patients of the colchicine group (13%) and 3 controls discontinued treatment (10%) at a median of 3 days after surgery. The main complaints associated with colchicine use were, as expected, gastrointestinal, with 5 patients (16.7%) in the colchicine group reporting diarrhea versus 1 control (3.4%) (p = 0.195). No serious complications in regard to renal and hepatic functions were observed and no cases of myotoxicity or myelotoxicity, either, which was to be expected given the short duration of treatment and the relatively low dose.
| Variable | Control (N=29) | Colchicine (N=30) | p value |
|---|---|---|---|
| Age (years) | 65.6±9.5 | 64.9±10.1 | 0.790 |
| Men | 20 (69%) | 21 (70%) | 0.931 |
| Weight (kg) | 80.0 [66.0-89.0] | 74.5 [69.8-88.3] | 0.654 |
| Body mass index (kg/m 2 ) | 27.6 [24.6-31.9] | 26.5 [24.1-28.4] | 0.355 |
| Body surface area (m 2 ) | 1.93±0.23 | 1.91±0.21 | 0.854 |
| Hypertension | 25 (86%) | 23 (77%) | 0.347 |
| Diabetes mellitus | 11 (38%)) | 14 (47%) | 0.497 |
| Dyslipidemia | 23 (79%) | 23 (77%) | 0.807 |
| Smoking | 15 (52%) | 14 (47%) | 0.698 |
| Past ST-elevation myocardial infarction | 5 (17%) | 6 (20%) | 0.786 |
| Prior percutaneous coronary intervention | 7 (24%) | 6 (20%) | 0.701 |
| Baseline estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 88±26 | 91±31 | 0.746 |
| Ejection fraction (%) | 50.0 [39.5-60.5] | 51.0 [43.0-61.5] | 0.654 |
| Euroscore II (%) | 2.34±0.99 | 2.21±0.96 | 0.616 |
| Baseline high-sensitivity troponin T (pg/ml) | 16.3±9.1 | 14.0±7.5 | 0.292 |
| Baseline creatine kinase-MB (ng/ml) | 3.1±1.6 | 2.7±1.5 | 0.266 |
| Number of grafted vessels | 3 [2-3] | 3 [2-3] | 0.980 |
| Extracorporeal time (min) | 121±41 | 119±35 | 0.850 |
| Aortic cross-clamping time (min) | 72±32 | 68±31 | 0.631 |
| Transfused packed red blood cell units | 1 [1-2] | 1 [1-2] | 0.420 |
| Maximal post-operative serum creatinine (mg/dl) | 1.2±0.3 | 1.1±0.2 | 0.172 |
| Maximal post-operative leukocyte count (10 6 /l) | 17.6±3.2 | 17.0±2.8 | 0.451 |
| Maximal post-operative C-reactive protein (mg/l) | 15.9±6.2 | 14.7±6.8 | 0.482 |
| Intensive care unit stay (days) | 2 [2-3] | 2 [2-2] | 0.082 |
| Treatment | |||
| β-blocker | 24 (83%) | 24 (80%) | 0.786 |
| ACEi/ARB | 14 (48%) | 18 (60%) | 0.366 |
| Calcium channel blocker | 5 (17%) | 6 (20%) | 0.786 |
| Diuretic | 9 (31%) | 14 (47%) | 0.218 |
| Statin | 24 (83%) | 22 (73%) | 0.383 |
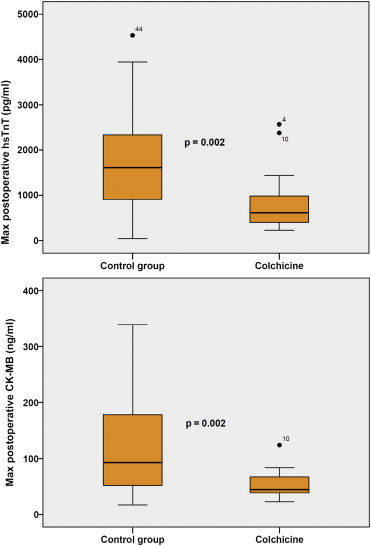
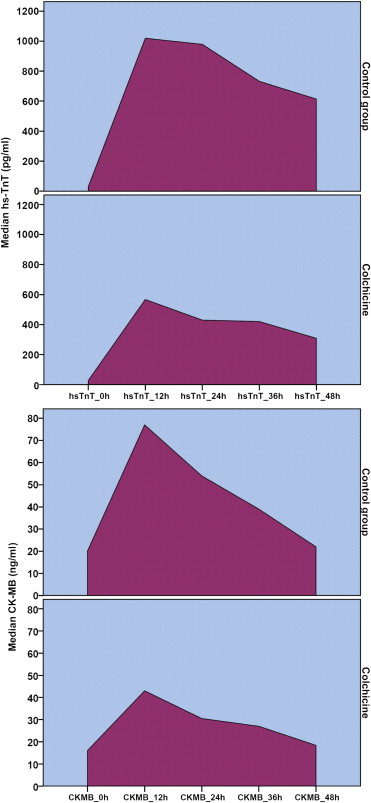
Maximal postoperative hsTnT levels were 616 pg/ml (396 to 986) in the colchicine group compared with 1,613 pg/ml (732 to 2,587) in controls (p = 0.002). Correspondingly, maximal CK-MB concentrations were 44.6 ng/ml (36.6 to 68.8) and 93.0 ng/ml (48.0 to 182.3), respectively (p = 0.002; Figure 1 ). The AUCs of postoperative myocardial injury biomarkers also differed significantly between the 2 groups. The median AUC for hsTnT was 40,755 pg h/ml (20,868 to 79,176) in controls versus 20,363 pg h/ml (13,891 to 31,661) in patients who took colchicine (p = 0.002). The corresponding areas for CK-MB were 2,552 ng h/ml (1,564 to 4,791) in controls compared with 1,586 ng h/ml (1,159 to 2,073) in the colchicine group (p = 0.003). Representative curves with the corresponding AUCs, illustrating the difference between the 2 groups, are shown in Figure 2 .
In the multivariable analysis (linear regression with the natural logarithm of maximal hsTnT as the dependent variable), the association of treatment allocation to the outcome parameter was adjusted for intraoperative hsTnT levels, time on extracorporeal circulation, and Euroscore II. The association of colchicine treatment with lower log(hsTnT) remained significant in this predefined multivariable model ( Table 2 ; model 1). Other models were also constructed, including factors such as gender, diabetes, ejection fraction, and ST-segment elevation myocardial infarction history, without any significant change to the explanatory power of the model or the significance of the association of treatment to log(hsTnT) (one of these exploratory models is listed as an example in Table 2 ; model 2).



