We sought to assess whether multiple biomarkers would correlate with the outcome and could improve event prediction in non–ST-segment elevation acute coronary syndrome populations with low event rates. Nine inflammatory, ischemic, or neurohormonal biomarkers were measured within 48 hours after symptom onset in 440 patients with non–ST-segment elevation acute coronary syndrome from the ARCHIPELAGO (Irbesartan in Patients With Acute Coronary Syndrome Without ST Segment Elevation) trial. We assessed the relation between biomarkers and ischemic or heart failure composite end points at 2 months of follow-up. We also evaluated whether biomarkers could improve the predictive performance of the validated and well-performing Global Registry of Acute Coronary Events risk score. Among all biomarkers measured at baseline, only interleukin-6 correlated with the ischemic end point (adjusted odds ratio 1.69, 95% confidence interval [CI] 1.23 to 2.31). The independent correlates of the heart failure end point were B-type natriuretic peptide (adjusted odds ratio 3.16, 95% CI 1.99 to 5.03), aldosterone (adjusted odds ratio 1.57, 95% CI 1.14 to 2.16) and matrix metalloproteinase-9 (adjusted odds ratio 0.64, 95% CI 0.46 to 0.88). The Global Registry of Acute Coronary Events score predicted poorly the ischemic end point (area under the curve [AUC] 0.591) and fairly (AUC 0.775) the heart failure end point. The performance of the models was significantly improved by the introduction of interleukin-6 (AUC 0.685) for the ischemic end point and of the 3 biomarkers (AUC 0.874) for the heart failure end point. In conclusion, the interleukin-6 level only, and B-type natriuretic peptide, aldosterone, and matrix metalloproteinase-9 together, independently correlated with the ischemic and heart failure end points, respectively. The Global Registry of Acute Coronary Events risk score’s performance was significantly improved with a biomarker strategy. In low-risk populations, a strategy using these biomarkers might help in identifying patients at greater risk of additional events.
The Global Registry of Acute Coronary Events (GRACE) score, considered one of the best performing scores, is based on simple admission clinical and biologic parameters, including age, heart rate, systolic blood pressure, plasma creatinine level, congestive heart failure, cardiac arrest, ST-segment segment deviation and elevated cardiac markers. Although the GRACE risk score provides an accurate estimation of in-hospital and 6-month probabilities of death and death or myocardial infarction in all coming patients with acute coronary syndrome (ACS), its discriminant value might be limited in the setting of low-risk patients.
Several biomarkers not considered in the previous scores have shown more or less controversial relations with the outcome in patients with ACS. Such markers include inflammatory or ischemic markers, such as C-reactive protein, interleukin-6 (IL-6), myeloperoxidase, ischemia-modified albumin, and secretory type II phospholipase-2 ; structural enzymes, such as matrix metalloproteinase (MMP-9) ; markers of platelet activation, such as CD40 − ligand ; and neurohormones, such as B-type natriuretic peptide (BNP) and aldosterone.
These markers are not used to stratify patients in routine practice. Although scores can be used to identify high-risk patients well, we wondered whether additional improvement in stratification could be obtained using biomarkers in a population with low event rates. We used the ARCHIPELAGO (Irbesartan in Patients With Acute Coronary Syndrome Without ST Segment Elevation) database, a randomized study that enrolled patients with non–ST-segment elevation acute coronary syndrome (NSTE-ACS) and subsequent low in-hospital (0.5%) and 2-month mortality (1.1%) rates, to assess the potential improvement of the risk prediction of models incorporating such markers in addition to the GRACE admission score.
Methods
ARCHIPELAGO was a multicenter randomized double-blind, double-dummy, 2 × 2 factorial, phase IIIb, 2-month study in which patients were randomized in a 1:1 ratio to either irbesartan 150 mg/d on admission to the hospital (early initiation) or at hospital discharge (late initiation) followed by irbesartan 300 mg/d from day 15 to day 60 (study end) or to enalapril 10 mg/d started early or late, force-titrated to 20 mg/d from Day 15 onward. The main objective of the study was to compare the effects of irbesartan and enalapril on the changes in the levels of high-sensitivity C-reactive protein in patients hospitalized with NSTE-ACS, and to evaluate whether a benefit was present for early or late initiation of treatment.
The randomized trial, the details of which have been previously published, failed to show any difference between the studied groups.
Adults aged ≥18 years who were admitted with ischemic symptoms (last episode within 48 hours before randomization) and ST or T changes in ≥2 leads or positive troponin test results were eligible for the study. The major exclusion criteria were persistent ST-segment elevation, coronary angiography or angioplasty planned before baseline sampling, creatinine clearance of ≤30 ml/min, congestive heart failure with New York Heart Association class III or IV symptoms, angioplasty, systolic blood pressure <100 mm Hg, and inflammatory or infectious disease or anti-inflammatory treatment.
Blood samples were drawn through venopuncture after randomization into tubes containing ethylenediaminetetraacetic acid, immediately centrifuged and stored at −80°C. The plasma levels of high-sensitivity C-reactive protein, IL-6, myeloperoxidase, secretory type II phospholipase-22, MMP-9, ischemia-modified albumin, sCD40-l, BNP, aldosterone, and troponin IC were assessed in a central laboratory using commercially available assays.
After randomization, the patients were clinically evaluated at discharge (or day 7 if not yet discharged) and days 15 and 60 ± 7. All events were assessed at each point, and we used the following definitions for the present study.
Death included mortality of any cause. Myocardial infarction was defined as ischemic symptoms with ST-segment elevation or new Q waves or left bundle branch block and an increase in creatine kinase-MB fraction or troponin levels >3 times the local upper limit of normal. Recurrent ischemia was defined as ischemic symptoms with ischemia-compatible ST-T changes and/or an increase in the troponin or creatine kinase-MB fraction levels greater than the local upper limit of normal. Stroke was defined by the presence of a new focal neurologic deficit thought to be of vascular origin, with signs or symptoms lasting >24 hours. Unplanned revascularization was defined as any coronary revascularization that was not planned during the initial hospitalization. Heart failure was defined as any increase of ≥1 point of the New York Heart Association class leading to a treatment modification (eg, initiation or dose increase of diuretics or inotropic agents or discontinuation or dose adjustment of β blockers), or a BNP level at the last follow-up visit (2 months) greater than 162 pg/ml, a value that has been validated in the diagnosis of heart failure.
We used 2 composite end points for the study, both assessed at the 2-month follow-up point: (1) the ischemic end point (the composite of death, stroke, nonfatal myocardial infarction, recurrent ischemia, or unplanned revascularization) and (2) the heart failure end point (the composite of death or heart failure).
All events occurring from inclusion to the end of study at 2 months of follow-up were reported and considered in the analysis only at their first occurrence.
The baseline characteristics were analyzed using the chi-square test or Fisher’s exact test and the Student t test or Mann-Whitney Wilcoxon test, as appropriate, for comparisons of variables between groups defined by the presence or absence of the end points.
The studied biomarkers all had skewed distributions and were divided into quartiles for additional analysis. The trends for the unadjusted analysis of event distribution according to biomarker quartiles were evaluated using a Cochran-Armitage trend test.
The association between biomarkers significantly associated with the coprimary end points in the univariate analysis was further assessed using logistic regression models. The multivariate model was systematically adjusted on the GRACE risk score for NSTE-ACS at admission. A 3-step analysis was performed introducing, successively, the GRACE score alone, the GRACE score and each of the biomarkers, and finally the GRACE score and all related biomarkers. Odds ratios were calculated for each quartile increment. Models were evaluated for their predictive performance using the area under the receiver operating characteristic curve (AUC). The 95% confidence intervals (CIs) were calculated using the bootstrapping procedure (n = 1,000 bootstrap samples) for AUC estimations and the differences between the models’ AUCs.
All numeric variables with a Gaussian distribution are presented as the mean ± SD, and those with a non-Gaussian distribution are presented as the median and interquartile range. Categorical variables are presented as the number and percentages. A p value <0.05 was considered significant. Analyses were performed using the Statistical Analysis Systems software package, version 9.1.3 (SAS Institute, Cary, North Carolina).
Results
Patients were included in the study 0.9 ± 0.3 days after the occurrence of symptoms. A total of 440 patients were randomized in the ARCHIPELAGO randomized trial, of whom 429 received ≥1 dose of the study medication. Figure 1 depicts the flow chart of the study. The global population of the present study consisted of the 419 patients (95%) with complete follow-up 60 ± 12 days after randomization.
As listed in Table 1 , most patients presented with non–ST-segment elevation myocardial infarction (77%), and 43% had ST-segment deviation. The patients mostly had a low (score <88, 74%) or intermediate (score 89% to 118%, 24%) risk of death or myocardial infarction, as assessed by the GRACE admission risk score, with only 2% in the highest risk group (score >118).
| Characteristic | Total (n = 440) | Ischemic End Point (n = 46) | Heart Failure End Point (n = 49) |
|---|---|---|---|
| Age (years) | 62 ± 14 | 65 ± 11 ⁎ | 72 ± 9 † |
| Men | 324 (74%) | 37 (80%) | 30 (61%) ⁎ |
| Reported and/or treated systemic hypertension | 203 (46%) | 22 (48%) | 32 (65%) ⁎ |
| Diabetes | 59 (13%) | 7 (15%) | 15 (31%) † |
| Reported and/or treated hypercholesterolemia | 213 (49%) | 20 (44%) | 22 (45%) |
| Current smoker | 155 (35%) | 15 (33%) | 7 (14%) ‡ |
| Body mass index (kg/m 2 ) | 27.2 ± 4.3 | 27.8 ± 4.1 | 28 ± 7 |
| Previous myocardial infarction | 52 (12%) | 5 (11%) | 11 (23%) ‡ |
| Previous coronary revascularization | 63 (14%) | 4 (9%) | 13 (27%) ‡ |
| Previous stroke | 19 (4%) | 5 (11%) ⁎ | 2 (4%) |
| Symptomatic peripheral vascular disease | 27 (6%) | 2 (5%) | 5 (10%) |
| Heart rate on admission (beats/min) | 67 ± 11 | 67 ± 11 | 75 ± 15 ⁎ |
| Systolic blood pressure (mm Hg) | 127 ± 18 | 132 ± 18 | 133 ± 19 |
| Diastolic blood pressure (mm Hg) | 73 ± 10 | 73 ± 13 | 75 ± 12 |
| Left ventricular ejection fraction (%) | 60 ± 11 | 60 ± 13 | 55 ± 14 |
| ST-segment deviation | 189 (43%) | 19 (41%) | 26 (54%) |
| Troponin IC level greater than upper limit of normal | 337 (77%) | 38 (86%) | 40 (82%) |
| Creatinine level (mg/dl) | 0.97 ± 0.3 | 1.01 ± 0.2 | 1.1 ± 0.3 |
| Global Registry of Acute Coronary Events score at admission | 91 ± 26 | 100 ± 27 ⁎ | 114 ± 23 † |
| Global Registry of Acute Coronary Events score at discharge | 101 ± 27 | 110 ± 27 ⁎ | 124 ± 23 † |
| Therapy before blood sample taken | |||
| Aspirin | 413 (96%) | 44 (96%) | 48 (98%) |
| Clopidogrel | 311 (72%) | 33 (72%) | 33 (67%) |
| Glycoprotein IIb/IIIa inhibitor | 92 (21%) | 9 (20%) | 9 (18%) |
| Low-molecular-weight heparin | 335 (78%) | 35 (76%) | 36 (74%) |
| Unfractionated heparin | 82 (19%) | 6 (13%) | 9 (18%) |
| Statin | 317 (73%) | 32 (70%) | 31 (63%) |
| β Blocker | 326 (75%) | 33 (72%) | 37 (76%) |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 35 (8%) | 5 (11%) | 10 (21%) |
| Troponin IC (ng/ml) | 0.8/2.8 | 1.1/4.2 | 1.5/3.8 |
| Aldosterone (pg/ml) | 47/52.4 | 48/57 | 68.5/77 ⁎ |
| B-type natriuretic peptide (pg/ml) | 83.5/120 | 91/119 | 206/234 † |
| CD40 ligand (ng/ml) | 3/4 | 3/4 | 2/3 |
| High-sensitivity C-reactive protein (mg/ml) | 4.2/9.4 | 4.1/15.9 | 4.8/16.2 |
| Interleukin-6 (pg/ml) | 6.4/10.7 | 9.5/14.8 ‡ | 8.7/8.3 |
| Ischemia-modified albumin (pg/ml) | 95/20 | 99/17 | 94/18 |
| Matrix metalloproteinase-9 (ng/ml) | 52.8/43.5 | 55.4/41.2 | 42.4/44.1 ⁎ |
| Myeloperoxidase (ng/ml) | 31.9/2022.6 | 32.3/20.3 | 30.7/17.2 |
| Secretory type II phospholipase-22 (ng/ml) | 2.5/3.6 | 2.6/2.5 | 3.3/8.6 |
‡ p <0.01 for patients with versus without end point at 2 months of follow-up.
The in-hospital course was relatively uneventful, with death reported in 2 patients (0.5%; both of cardiovascular origin), nonfatal ST-segment elevation acute myocardial infarction in 2 patients (0.5%), unplanned revascularization in 5 patients (1.1%), and heart failure in 3 patients (0.7%). As reported in Table 2 , most patients (86%) underwent coronary angiography, and 42% underwent revascularization. The in-hospital management and treatments at discharge did not differ between groups when defined by the occurrence or absence of the end points. The treatment at discharge was optimal, with a large majority of patients treated with dual antiplatelet therapy (statins and β blockers).
| Characteristic | Total (n = 440) | Ischemic End Point (n = 46) | Heart Failure End Point (n = 49) |
|---|---|---|---|
| Coronary angiography | 356 (81%) | 36 (78%) | 36 (73%) |
| Percutaneous coronary intervention | 145 (33%) | 12 (26%) | 15 (30%) |
| Coronary artery bypass grafting | 41 (9%) | 6 (13%) | 6 (12%) |
| Therapy at discharge | n = 438 | n = 44 | n = 47 |
| Aspirin | 400 (91%) | 43 (98%) | 47 (100%) |
| Clopidogrel | 353 (80%) | 42 (95%) | 41 (87%) |
| Statin | 317 (72%) | 40 (90%) | 40 (85%) |
| β Blocker | 347 (79%) | 38 (86%) | 42 (89%) |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 429 (98%) | 44 (100%) | 47 (100%) |
From discharge to the follow-up point, the following events were reported: death in 3 (0.7%), nonfatal myocardial infarction in 11 (2.6%), stroke in 1 (0.2%), recurrent ischemia in 13 (3.1%), and unplanned coronary intervention in 13 (3.1%). One episode of acute heart failure occurred in 1 patient (0.2%), and subclinical heart failure, as defined by a BNP level >162 pg/ml at the 2-month visit, was reported for 43 patients (10%).
At 2 months of follow-up, the composite ischemic and heart failure end points had occurred in 46 (11%) and 49 (12%) patients, respectively, including the in-hospital events.
Compared to those not meeting the end point, the patients in whom the ischemic end point was reported were significantly older (65 ± 11 vs 61 ± 11 years, p <0.05) and more often had a history of ischemic stroke (11% vs 3%, p <0.05) and a greater admission GRACE score (100 ± 27 vs 91 ± 26, p <0.05).
Patients who met the heart failure composite end point were significantly older (72 ± 9 vs 60 ± 12 years, p <0.001) and were less often men (61% vs 76%, p <0.05) compared to those not meeting the end point. They also had greater rates of systemic hypertension (65% vs 44%, p <0.05), diabetes (31% vs 11%, p <0.0001), a history of myocardial infarction (23% vs 10%, p <0.01), and coronary revascularization (27% vs 14%, p <0.05) and a greater admission GRACE score (114 ± 23 vs 86 ± 25, p <0.0001) but lower rates of current smoking (14% vs 37%, p <0.01).
Only the IL-6 level was significantly greater in the patients reaching the composite ischemic end point than in the patients without an event (median 9.5, interquartile range 14.8, vs median 6.1, interquartile range 10.1 pg/ml; p = 0.002). Those reaching the composite heart failure end point had greater admission BNP levels (median 206, interquartile range 234, vs median 74, interquartile range 90; p <0.0001), aldosterone (median 68.5, interquartile range 77, vs median 44.7, interquartile range 47.7; p = 0.02) and lower levels of MMP-9 (median 42.4, interquartile range 44.1, vs median 55, interquartile range 44; p = 0.01).
The distribution of event rates according to biomarker quartiles is shown in Figure 2 .
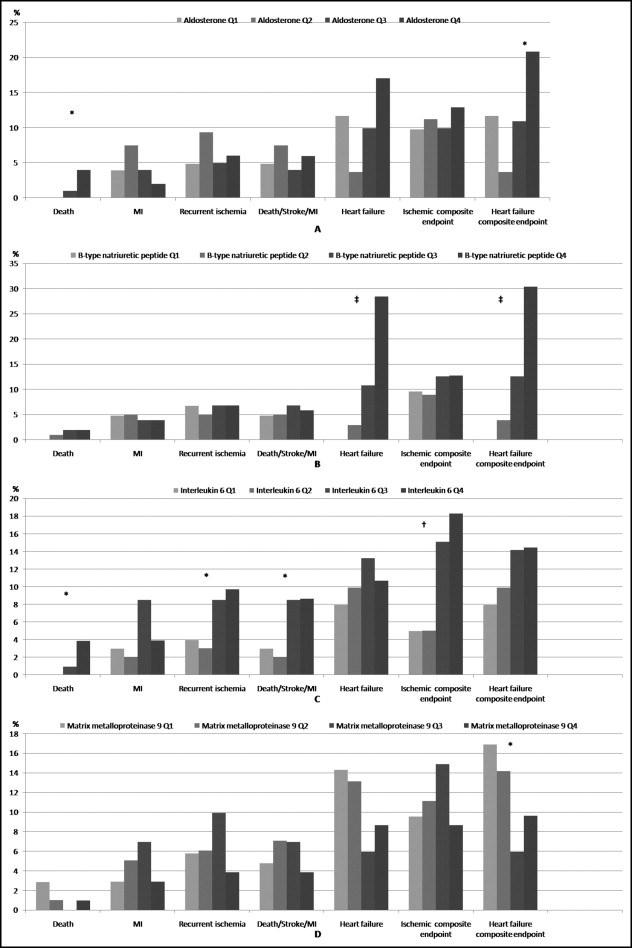
A significant relation was found between death and the aldosterone, but not BNP or MMP-9, levels. None of the previous biomarkers correlated with the ischemic events.
The IL-6 level consistently correlated with the occurrence of death, recurrent ischemia, the composite of death, stroke, and myocardial infarction, and the composite ischemic end point.
The unadjusted odds ratio per quartile increment was 1.74 (95% CI 1.28 to 2.37) for IL-6 in relation to the composite ischemic end point and 3.68 (95% CI 2.41 to 5.64) for BNP, 1.41 (95% CI 1.07 to 1.89) for aldosterone, and 0.74 (95% CI 0.56 to 0.97) for MMP-9 in relation to the composite heart failure end point. The distribution of the heart failure end point based on aldosterone and MMP-9 quartiles was not stepwise.
The stepwise multivariate logistic regression ( Table 3 ) revealed the persistence of a significant association between all biomarkers and related end points after adjustment for the GRACE score and/or other biomarkers.



