We investigated the efficacy of short-term high-dose atorvastatin in decreasing the risk of contrast-induced nephropathy (CIN) in patients with chronic kidney disease (CKD) subjected to coronary angiography and/or angioplasty. CIN occurs in up to 15% of patients with pre-existing CKD and affects clinical outcome. The protective effect of statin therapy against CIN is still controversial. A prospective, single-center study of 304 patients with baseline estimated creatinine clearance <60 ml/min were randomized to receive atorvastatin 80 mg/day or placebo for 48 hours before and 48 hours after contrast medium administration. All patients received intravenous saline hydration and oral N -acetylcysteine 1,200 mg 2 times/day. Iso-osmolar contrast medium was used. CIN was defined as an absolute increase of serum creatinine ≥0.5 mg/dl within 5 days after the procedure. CIN occurred in 31 patients (10%), 16 (11%) in the placebo group and 15 (10%) in the atorvastatin group (p = 0.86). Mean increase in creatinine was not significantly different in the 2 groups (0.59 ± 0.17 in placebo group vs 0.72 ± 0.26 mg/dl in atorvastatin group, p = 0.31). Persistent kidney injury, defined as 1-month increase from baseline creatinine value ≥25%, was observed in 30% in the placebo group and in 31% in the atorvastatin group (p = 0.58). In conclusion, a short-term administration of high doses of atorvastatin before and after contrast exposure, in addition to standard intravenous hydration and oral N -acetylcysteine, does not decrease CIN occurrence in patients with pre-existing CKD.
Contrast-induced nephropathy (CIN) is an important possible complication after contrast angiography that occurs in up to 15% of patients with pre-existing chronic kidney disease (CKD). This early postprocedural event can prolong hospitalization, produce persistent worsening of renal function, and is associated with poor long-term clinical outcome. Given the complexity of the underlying pathophysiologic mechanisms several different protocols have been tested in an effort to prevent onset of CIN. Certain of these prophylactic strategies have become routine practice for preventing CIN, for example, prophylactic intravenous volume expansion with isotonic crystalloid solution, whereas others are still under investigation, including the choice of intravenous saline or sodium bicarbonate solution, antioxidant therapy with oral N -acetylcysteine (NAC) or ascorbic acid, and use of low- or iso-osmolarity contrast agents. Statins have recently been proposed for prevention of CIN given their antioxidant and anti-inflammatory properties. Different specific studies have produced conflicting results. The aim of this study was to evaluate the efficacy of administration of short-term high-dose atorvastatin, added to routine intravenous hydration and oral NAC, in decreasing the occurrence of CIN in patients with CKD undergoing planned coronary angiography and/or intervention.
Methods
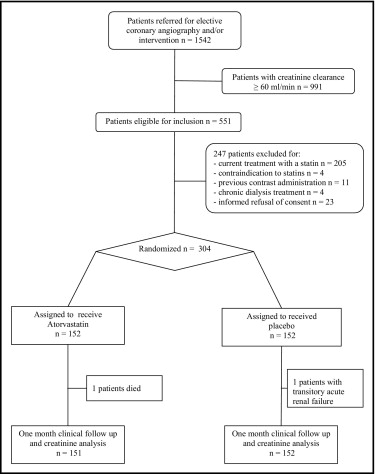
This was a prospective, randomized, placebo-controlled trial performed in patients with baseline CKD undergoing elective coronary angiography and/or other intervention. From April 2006 to March 2008, 1,542 patients underwent planned coronary angiographic procedures at our institution. Of these, 551 patients had a preangiographic estimated creatinine clearance <60 ml/min—evaluated by applying the Cockcroft-Gault formula —and were considered eligible for inclusion in our study. Exclusion criteria were current treatment with a statin, contraindication to statin treatment, previous contrast media administration (within 10 days of study entry), end-stage renal failure requiring dialysis, and informed refusal of consent. Thus, 304 patients were enrolled in the study ( Figure 1 ). Randomization was performed by computerized open-label assignment in blinded envelopes used in a consecutive fashion; 152 patients were assigned to receive high doses of atorvastatin (80 mg/day) and 152 to receive matching placebo for 48 hours before and 48 hours after contrast medium administration. All patients received intravenous standard hydration with isotonic saline (0.9% sodium chloride 1 ml/kg/hour for 12 hours before and after the procedure) and oral NAC 1,200 mg 2 times/day from the day before to the day after the procedure. Hydration rate was decreased to 0.5 ml/kg/hour in the 2 study arms for patients with left ventricular ejection fraction ≤40%. The same nonionic, dimeric iso-osmolar contrast medium (iodixanol; Visipaque, GE Healthcare, Ltd., Amersham, United Kingdom) was used in all cases.
Serum creatinine concentration was assessed at the time of hospital admission and on days 1, 2, 3, 5, and 10 after the procedure. A further measurement was performed at 1 month in all cases of CIN. All tests were performed in the same hospital laboratory with consistent methods. All patients presented for creatinine determination and clinical follow-up at 1 month. Data were recorded in a dedicated database.
Renal function was classified according to the stages set by the National Kidney Foundation (USA), with creatinine clearances ≥90 ml/min considered normal, 60 to 89 ml/min mildly impaired, 30 to 59 ml/min moderately impaired, and <30 ml/min severely impaired. The CIN risk score was calculated as specified by Mehran et al. High-contrast load was defined as an administered contrast volume ≥140 ml. CIN was defined as an absolute serum creatinine increase of ≥0.5 mg/dl over baseline within 5 days after the administration of radiographic contrast medium. A difference of ≥25% in creatinine values from baseline to 1 month after intervention was defined as persistent impairment of renal function.
The protocol was approved by the hospital ethics committee and all patients gave written informed consent. The study was not supported by any external source of funding.
The primary end point was the development of CIN defined as an absolute serum creatinine increase ≥0.5 mg/dl over baseline within 5 days after contrast agent administration. Additional end points were (1) development of CIN defined as a relative serum creatinine increase ≥25% over baseline within 5 days and (2) adverse clinical events within 1 month, including in-hospital death and need for dialysis or hemofiltration.
The sample size was calculated by assuming a 15% incidence of the study end point in the placebo group; 300 patients would be required (150 per treatment group) to detect a 50% relative decrease in the incidence of the end point in the atorvastatin group with 90% power at the conventional, 2-sided significance level of 5%. Statistical analysis was performed with SPSS 13.0 (SPSS, Inc., Chicago, Illinois). Categorical variables were presented as counts and percentages and compared by chi-square or Fisher’s exact test. Continuous variables were compared by Student’s t test for normally distributed values; Mann-Whitney U test was used for other variables. All p values are 2-tailed and statistical significance was defined as a p value <0.05.
Results
The median age for the entire study cohort was 75 years (interquartile range 71 to 81). Mean baseline estimated creatinine clearance was 46 ± 10 ml/min and 8% of patients had severe renal impairment with estimated creatinine clearance <30 ml/min. There were no significant differences in baseline clinical, biochemical, and procedural characteristics between the atorvastatin and placebo groups ( Table 1 ).
| Variable | Placebo Group | Atorvastatin Group | p Value |
|---|---|---|---|
| (n = 152) | (n = 152) | ||
| Age (years) | 76 ± 7 | 75 ± 8 | 0.44 ⁎ |
| Body mass index (kg/m 2 ) | 25 ± 3 | 26 ± 4 | 0.27 ⁎ |
| Women | 60 (40%) | 48 (32%) | 0.38 † |
| Hypertension | 89 (59%) | 95 (63%) | 0.80 † |
| Diabetes mellitus | 33 (22%) | 31 (20%) | 0.79 † |
| Known coronary artery disease | 44 (29%) | 47 (31%) | 0.42 † |
| Serum creatinine (mg/dl) | 1.18 ± 0.33 | 1.20 ± 0.35 | 0.68 ⁎ |
| Serum creatinine ≥1.5 mg/dl | 22 (15%) | 22 (15%) | 0.95 † |
| Estimated creatinine clearance (ml/min) | 46 ± 11 | 46 ± 10 | 0.94 ⁎ |
| Estimated creatinine clearance <30 ml/min | 14 (9%) | 10 (7%) | 0.47 † |
| Serum urea nitrogen (mg/dl) | 43 ± 18 | 46 ± 19 | 0.18 ⁎ |
| Proteinuria (mg/dl) | 15 ± 31 | 11 ± 15 | 0.47 ⁎ |
| Left ventricular ejection fraction (%) | 47 ± 11 | 49 ± 9 | 0.25 ⁎ |
| Left ventricular ejection fraction ≤40% | 49 (32%) | 41 (27%) | 0.44 † |
| Angiotensin-converting enzyme inhibitors | 44 (29%) | 45 (30%) | 0.95 † |
| Diuretic therapy | 52 (34%) | 51 (36%) | 0.92 † |
| Hemoglobin (mg/dl) | 13.0 ± 1.5 | 13.5 ± 1.5 | 0.12 ⁎ |
| Hematocrit (%) | 37.4 ± 4.7 | 38.9 ± 4.4 | 0.10 ⁎ |
| Total cholesterol (mg/dl) | 183.9 ± 35 | 190.6 ± 43 | 0.10 ⁎ |
| High-density lipoprotein cholesterol (mg/dl) | 40.8 ± 11 | 44.1 ± 13 | 0.10 ⁎ |
| Low-density lipoprotein cholesterol (mg/dl) | 120.5 ± 30 | 121.4 ± 31 | 0.10 ⁎ |
| Triglycerides (mg/dl) | 121.5 ± 66 | 110.4 ± 35 | 0.10 ⁎ |
| Coronary angiography/elective percutaneous coronary intervention | 68 (45%)/84 (55%) | 75 (50%)/77 (50%) | 0.55 † |
| Mean contrast volume administered (ml) | 164 ± 99 | 151 ± 95 | 0.30 ⁎ |
| Contrast medium volume ≥140 ml | 80 (53%) | 71 (47%) | 0.41 † |
| Contrast nephropathy risk score | |||
| Mean score | 10 ± 3 | 9 ± 3 | 0.10 ⁎ |
| Score ≤5 | 25 (16%) | 28 (18%) | 0.85 † |
| Score 6–10 | 62 (41%) | 67 (44%) | |
| Score 11–16 | 60 (40%) | 53 (35%) | |
| Score ≥17 | 5 (3%) | 4 (3%) |
⁎ Values were compared using unpaired t test.
† Values were compared using chi-square or Fisher’s exact test.
Mean creatinine values are listed in Table 2 . No differences were found in baseline and follow-up creatinine levels between the 2 groups at any time. Overall, creatinine increased significantly after contrast medium administration (baseline 1.19 ± 0.54 vs peak 1.27 ± 0.42, p = 0.001), but the mean absolute increase was not significantly different in the 2 groups (0.09 ± 0.23 mg/dl in placebo group vs 0.07 ± 0.30 mg/dl in atorvastatin group, p = 0.51).
| Variable | Placebo Group | Atorvastatin Group | p Value ⁎ |
|---|---|---|---|
| (n = 152) | (n = 152) | ||
| Serum creatinine (mg/dl) | |||
| Baseline | 1.18 ± 0.33 | 1.20 ± 0.35 | 0.68 |
| Day 1 after angiography | 1.16 ± 0.34 | 1.13 ± 0.35 | 0.52 |
| Day 2 after angiography | 1.19 ± 0.39 | 1.18 ± 0.36 | 0.70 |
| Day 3 after angiography | 1.21 ± 0.42 | 1.21 ± 0.39 | 0.97 |
| Day 5 after angiography | 1.21 ± 0.39 | 1.19 ± 0.40 | 0.75 |
| Peak after angiography | 1.28 ± 0.42 † | 1.27 ± 0.42 † | 0.89 |
⁎ Values were compared using unpaired t test.
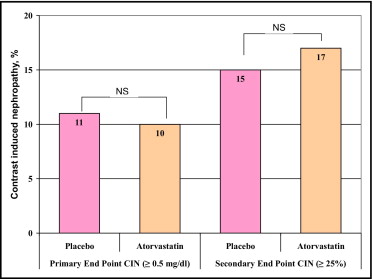
The primary CIN end point occurred in 31 patients (10%), 16 (11%) in the placebo group and 15 (10%) in the atorvastatin group (p = 0.86; Figure 2 ). No significant difference was observed between the 2 groups even when CIN was defined as ≥25% relative increase in baseline serum creatinine (15% in placebo group vs 17% in atorvastatin group, p = 0.67; Figure 2 ). Table 3 presents the incidence of CIN in high-risk patients with no significant differences between the 2 groups. Table 4 lists mean creatinine values in patients who developed CIN. No significant differences were found between the 2 groups at baseline and follow-up creatinine measurement. In patients who developed CIN, mean creatinine values remained significantly higher than baseline (1.56 ± 0.62 vs 1.39 ± 0.61 mg/dl, respectively, p = 0.001) at 1 month, without significant differences between the 2 groups; persistent renal injury was observed in 30% of patients in the placebo group and 31% in the atorvastatin group (p = 0.58).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


