Heart failure (HF) with preserved left ventricular ejection fraction (LVEF) and diabetes commonly coexist, but the impact of diabetes on HF outcomes in patients with HF and preserved LVEF has not been well studied. We assessed the risk of HF death or hospitalization for worsening HF associated with diabetes by studying 987 patients with HF and preserved LVEF enrolled in the Digitalis Investigation Group (DIG) ancillary study. Diabetics (n = 285, 28.9%) were younger, had a larger body mass index, faster heart rate, and higher pulse pressure than nondiabetics. Diabetics were also more likely to be women, have a history of hypertension, ischemic cause for HF, and were more likely to be treated with diuretics. During the mean follow-up of 37 months, 88 (30.9%) diabetics and 133 (19.0%) nondiabetics developed the primary outcome of HF hospitalization or HF death. After adjustments for baseline differences, diabetes was associated with a 68% increased risk of HF hospitalization or HF death (adjusted hazard ratio 1.68, 95% confidence interval 1.26 to 2.25, p <0.001). In conclusion, in patients with HF and preserved LVEF, diabetes is associated with significantly increased risk of developing adverse HF outcomes.
Diabetes mellitus is an important co-morbidity likely contributing to adverse heart failure (HF) outcomes in patients with HF and preserved left ventricular ejection fraction (LVEF). The prevalence of diabetes continues to increase in the general population and in community-based patients with HF. Although diabetes has been shown to be a predictor of adverse cardiovascular outcomes in patients with decreased LVEF, the impact of diabetes on adverse HF outcomes has not been well studied in patients with HF and preserved LVEF. Given potential diabetes-associated effects on vascular compliance and LV systolic stiffness and potential effects on LV diastolic function, processes that have been implicated in the pathophysiology of HF with preserved LVEF, we hypothesized that diabetes would be associated with increased rates of hospitalization for worsening HF or HF death in this group of patients. Therefore, we examined the prognostic impact of diabetes on adverse HF outcomes in patients with preserved LVEF enrolled in the Digitalis Investigation Group (DIG) study.
Methods
The database used for this study was a public-use copy of the DIG study from the National Heart, Lung, and Blood Institute (Bethesda, Maryland). The design and primary analyses of the DIG study have been described in detail. Briefly, the DIG study tested the effects of effects of digoxin in mortality and hospitalization in patients with HF. The diagnosis of HF was based on current or previous clinical symptoms, signs, or radiologic evidence of pulmonary congestion. Important exclusion criteria included recent myocardial infarction or unstable angina (<4 weeks from enrollment) and renal insufficiency (defined as creatinine level >3.0 mg/dl). Patients were recruited from February 1991 through August 1993. Although the main results of the trial were published in the 6,800 patients with an LVEF ≤45%, the DIG study also included an ancillary trial of 988 patients with HF and an LVEF >45%. The results of digoxin therapy on cardiovascular outcomes in the patients with HF and preserved LVEF of the DIG ancillary trial were recently published. Our analyses are limited to these patients with HF and LVEF >45% who were enrolled in the DIG ancillary trial.
Baseline demographics were obtained at entry into the study. Classification of diabetes was based on a reported history of diabetes at study entry. Data on baseline diabetes were available in 987 participants; 1 patient did not have data available for the classification of diabetes and was excluded from the study. Diabetic treatment or classification between type 1 and type 2 diabetes was not available.
The primary outcome was time to hospitalization for worsening HF or HF death. Secondary outcomes included individual outcomes of times to hospitalization for worsening HF, HF mortality, all-cause mortality and cardiovascular mortality, and time to combined outcome of cardiovascular mortality and hospitalization for worsening HF. Differences in baseline variables were compared using chi-square tests for categorical variables and t tests for continuous variables. Two-sided p values <0.05 were considered statistically significant. Univariate and multivariable Cox proportional hazards models were used to assess the relation between diabetes and outcomes of interest. Covariates included variables identified as important predictors of the combined outcome of HF death or hospitalization for worsening HF, and variables with p values <0.10 by univariate analyses were entered into the multivariable model. The final model included age, gender, LVEF, cardiothoracic ratio, heart rate, diastolic blood pressure, brachial pulse pressure, number of signs or symptoms of HF, New York Heart Association (NYHA) classification, history of myocardial infarction, ischemic HF cause, previous digoxin use, nonpotassium-sparing diuretic use, glomerular filtration rate (GFR), and body mass index (BMI). GFR was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) Study Group equation. Brachial pulse pressure was calculated as the difference between brachial systolic and diastolic blood pressures. Tests for interaction between diabetes and subgroups (gender, age, ischemic cause of HF, obesity, and NYHA classification) were performed by adding a cross-product term of these variables on the combined primary outcome of HF death or hospitalization for worsening HF; the test for a statistical interaction between diabetes and subgroup were performed before other variables being entered into the model. Statistical analysis was performed using STATA 9.2 (STATA Corp., College Station, Texas).
Results
Of the total 987 patients with HF and an LVEF >45% included in this analysis, 285 patients (28.9%) had a documented history of diabetes. Baseline characteristics according to diabetic status are listed in Table 1 . At baseline, diabetic patients were younger and were more likely to be women, have a history of hypertension, have ischemic cause for HF, and were more likely to be treated with diuretics and nitrates. Diabetic patients also had a larger BMI, faster heart rate, had a higher systolic blood pressure and pulse pressure, and were more likely to have peripheral edema and radiologic evidence of pulmonary congestion than nondiabetic patients at baseline.
| Variable | Diabetes Mellitus | p Value | |
|---|---|---|---|
| Yes (n = 285) | No (n = 702) | ||
| Age (years) | 64.8 ± 9.8 | 67.6 ± 10.4 | <0.001 |
| Women | 48.8% | 38.0% | 0.002 |
| Nonwhite | 16.5% | 12.8% | 0.13 |
| Body mass index (kg/m 2 ) | 30.8 ± 7.3 | 27.7 ± 5.5 | <0.001 |
| Left ventricular ejection fraction (%) | 55.1 ± 7.9 | 55.6 ± 8.2 | 0.37 |
| Heart rate (beats/min) | 77.7 ± 12.4 | 75.0 ± 11.7 | 0.002 |
| Systolic blood pressure (mm Hg) | 141.4 ± 21.8 | 136.0 ± 20.9 | <0.001 |
| Diastolic blood pressure (mm Hg) | 76.5 ± 12.0 | 77.0 ± 11.1 | 0.58 |
| Brachial pulse pressure (mm Hg) | 64.8 ± 18.5 | 59.0 ± 18.1 | <0.001 |
| Signs and/or symptoms of heart failure | |||
| Rales | 74.7% | 73.2% | 0.62 |
| Increased jugular venous pressure | 50.5% | 46.3% | 0.23 |
| Peripheral edema | 69.5% | 55.0% | <0.001 |
| S 3 | 31.9% | 33.5% | 0.64 |
| Radiologic evidence of pulmonary congestion | 68.4% | 60.0% | 0.01 |
| Cardiothoracic ratio | 0.52 ± 0.08 | 0.52 ± 0.08 | 0.50 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 60.0 ± 20.8 | 62.4 ± 20.4 | 0.09 |
| Potassium (mmol/L) | 4.4 ± 0.4 | 4.3 ± 0.5 | 0.007 |
| Ischemic heart failure cause | 64.6% | 53.1% | 0.001 |
| New York Heart Association class III–IV | 24.2% | 21.1% | 0.29 |
| Previous myocardial infarction | 54.4% | 47.6% | 0.05 |
| Current angina | 33.7% | 28.2% | 0.09 |
| Hypertension | 70.9% | 55.1% | <0.001 |
| Previous digoxin use | 32.0% | 36.6% | 0.17 |
| Medication | |||
| Nonpotassium-sparing diuretic | 81.8% | 73.7% | 0.007 |
| Potassium-sparing diuretic | 7.4% | 8.3% | 0.64 |
| Angiotensin-converting enzyme inhibitors | 88.8% | 85.2% | 0.14 |
| Nitrates | 44.6% | 37.3% | 0.04 |
| Digoxin | 47.4% | 50.7% | 0.34 |
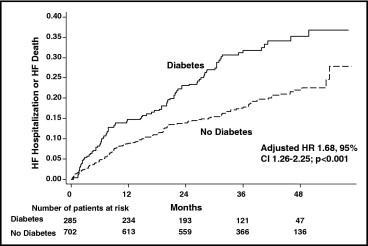
During the mean follow-up of 37 months, diabetic patients were more likely than nondiabetic patients to develop the primary outcome of HF mortality or hospitalization for worsening HF (unadjusted hazard ratio [HR] 1.79, 95% confidence interval 1.37 to 2.35, p <0.001; Figure 1 , Table 2 ). After adjusting for potential confounders, including age, gender, LVEF, cardiothoracic ratio, heart rate, diastolic blood pressure, brachial pulse pressure, number of signs or symptoms of HF, NYHA classification, history of myocardial infarction, ischemic HF cause, previous digoxin use, nonpotassium-sparing diuretic use, GFR, and BMI, the adjusted HR for the combined primary outcome of HF death or hospitalization for worsening HF was 1.68 (95% CI 1.26 to 2.25, p <0.001).
| Variable | Diabetes Mellitus | p Value | |
|---|---|---|---|
| Yes | No | ||
| (n = 285) | (n = 702) | ||
| Heart failure death or hospitalization for worsening heart failure | 88 (30.9%) | 133 (19.0%) | |
| Unadjusted hazard ratio (95% CI) | 1.79 (1.37–2.35) | reference | <0.001 |
| Adjusted hazard ratio (95% CI) ⁎ | 1.68 (1.26–2.25) | reference | <0.001 |
| Hospitalization for worsening heart failure | 81 (28.4%) | 116 (16.5%) | |
| Unadjusted hazard ratio (95% CI) | 1.88 (1.42–2.50) | reference | <0.001 |
| Adjusted hazard ratio (95% CI) | 1.76 (1.30–2.39) | reference | <0.001 |
| Heart failure death | 21 (7.4%) | 43 (6.1%) | |
| Unadjusted hazard ratio (95% CI) | 1.26 (0.75–2.12) | reference | 0.39 |
| Adjusted hazard ratio (95% CI) | 1.40 (0.78–2.50) | reference | 0.26 |
| Total mortality | 81 (28.4%) | 150 (21.4%) | |
| Unadjusted hazard ratio (95% CI) | 1.38 (1.06–1.81) | reference | 0.02 |
| Adjusted hazard ratio (95% CI) | 1.48 (1.10–1.99) | reference | 0.009 |
| Cardiovascular mortality | 58 (20.3%) | 104 (14.8%) | |
| Unadjusted hazard ratio (95% CI) | 1.43 (1.03–1.97) | reference | 0.03 |
| Adjusted hazard ratio (95% CI) | 1.54 (1.08–2.18) | reference | 0.02 |
| Cardiovascular mortality or hospitalization for worsening heart failure | 116 (40.7%) | 180 (25.6%) | |
| Unadjusted hazard ratio (95% CI) | 1.76 (1.39–2.22) | reference | <0.001 |
| Adjusted hazard ratio (95% CI) | 1.69 (1.32–2.17) | reference | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


