Platelet reactivity is greater in patients with stable angina and with more extensive peripheral vascular atherosclerosis. We sought to evaluate whether impaired peripheral microcirculatory endothelial function might correlate with platelet reactivity after clopidogrel and therefore predispose to an unfavorable outcome after percutaneous coronary intervention (PCI). In 52 consecutive patients with stable angina undergoing elective PCI, endothelial function was assessed by (1) endothelial peripheral arterial tonometry (measuring the “Endoscore”); (2) the von Willebrandt factor antigen level and ristocetin co-factor activity. Basal platelet reactivity was assessed by soluble P-selectin. Patients then received a 600-mg clopidogrel loading dose ≥12 hours before PCI. A blood sample was withdrawn 12 hours later, but before PCI, to assess platelet reactivity using the P2Y12 reaction unit and percentage of P2Y12 inhibition with the point-of-care VerifyNow P2Y12 assay. Troponin T was assessed 24 hours after PCI. The Endoscore inversely correlated with von Willebrandt factor antigen activity (r = −0.52, p = 0.0001) and soluble P-selectin concentration (r = −0.36, p = 0.021), suggesting greater platelet reactivity with increased impaired endothelial function. After clopidogrel, the Endoscore correlated directly with the percentage of P2Y12 inhibition (r = 0.36, p = 0.009) and inversely with the P2Y12 reaction unit (r = −0.41, p = 0.002), suggesting greater residual platelet reactivity with more impaired endothelial function. The average Endoscore was significantly lower in patients with troponin T elevation (troponin positive group 0.267 ± 0.091) than in patients without troponin T elevation (troponin negative group 0.508 ± 0.041, p = 0.015 vs troponin positive). In conclusion, an impaired endothelial response before clopidogrel was associated with greater platelet reactivity after clopidogrel. This link might explain the unfavorable PCI outcomes in patients with more severe endothelial impairment.
Dual antiplatelet therapy with aspirin and clopidogrel is being widely administered to patients undergoing percutaneous coronary intervention (PCI). However, high residual platelet reactivity can limit the benefit of clopidogrel in ≤21% of patients, resulting in increased cardiovascular events both during the procedural and during long-term follow-up. Genetic, cellular, and clinical factors might account for the suboptimal platelet response to clopidogrel. In addition, basal platelet reactivity is a strong predictor of the platelet response to clopidogrel. Platelet reactivity has been related to systemic atherosclerotic disease. In particular, greater platelet reactivity has been reported in the presence of more severe and diffuse peripheral vascular atherosclerosis. Whether the latter also has an effect on postclopidogrel platelet reactivity has not been reported. We tested the hypothesis that impaired peripheral endothelial function, an early marker of vascular atherosclerosis, correlates with platelet reactivity after the administration of clopidogrel.
Methods
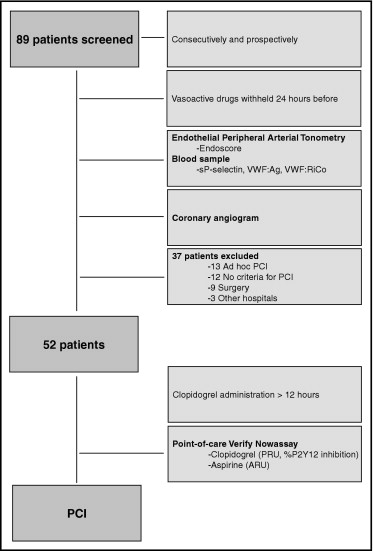
A total of 89 patients with stable angina who were scheduled to undergo elective coronary angiography were consecutively and prospectively assessed for peripheral endothelial function. Only those patients for whom PCI was electively staged (n = 52) were finally included in the study protocol. The reasons to not stage PCI were (1) ad hoc PCI (n = 13); (2) the absence of significant coronary stenosis at angiography (n = 12); (3) a referral for coronary bypass surgery (n = 9); and (4) treatment at another hospital (n = 3). The exclusion factors were the presence of acute coronary syndrome, a known allergy to clopidogrel, a history of gastrointestinal bleeding, or thrombocytopenia with a platelet count of <100/nl, pretreatment with clopidogrel, chronic renal failure (glomerular filtration rate <60 ml/min), inflammatory disease, serious co-morbidities, or a left ventricular ejection fraction <50%. All patients were receiving chronic aspirin treatment (160 to 325 mg) at recruitment.
The study protocol is shown in Figure 1 . All vasoactive medications were withheld for ≥24 hours before the measurements were taken. Peripheral endothelial function assessment and blood sample withdrawal (for endothelial and platelet biomarker measurement) were performed in the morning, after a 12-hour fasting and before coronary angiography. Thus, the investigators were unaware of the patients’ coronary artery disease and the possible treatment strategy. In patients with significant coronary lesions, PCI was scheduled such that a 600-mg loading dose of clopidogrel was given uniformly ≥12 hours before PCI. Except for clopidogrel, no changes in the ongoing medical therapy were allowed between the diagnostic and interventional procedures. Blood samples were withdrawn at PCI for assessment of platelet inhibition by clopidogrel. The PCIs were uncomplicated and performed according to the operator’s discretion. During catheterization, all patients received intravenous heparin to achieve a target activated clotting time of 250 to 350 seconds. No glycoprotein IIb/IIIa blockers were administered. An additional blood withdrawal was performed 24 hours after PCI for troponin T measurement. The local ethics committee approved the study protocol, and all patients provided written informed consent.
Peripheral endothelial function was measured by digital pulse amplitude with the Endothelial Peripheral Arterial Tonometry (Endo-PAT2000, Itamar Medical, Caesarea, Israel), as previously described. Figure 2 shows an example of the tracings obtained with the Endo-PAT2000 from 2 patients, 1 with good and 1 with poor endothelial function. In brief, the device measures the distal finger blood volume changes that accompany pulse waves. A peripheral arterial tonometry finger probe was placed at the tip of each index finger, and a blood pressure cuff was placed at the level of the study arm. After a 5-minute resting period (baseline), the blood pressure cuff was inflated to 20 mm Hg greater than the systolic pressure for 5 minutes (occlusion). Next, the blood pressure cuff was deflated, and the peripheral arterial tonometry recording was continued for an additional 5 minutes (hyperemia; Figure 2 ). The endothelial responses were assessed using the recently validated Framingham Reactive Hyperemia Index (Endoscore), according to the following formula: Endoscore = lan[RH occluded (90 to 120 seconds)/RH control (90 to 120 seconds)] =
l a n ( P W A o c c ( 90 s − 120 s ) P W A o c c ( B L ) ∕ P W A c o n t ( 90 s − 120 s ) P W A c o n t ( B L ) )
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


