Catheter-based treatment of pulmonary embolism (PE) has been demonstrated to be successful in case reports and small series. The investigators report the results of a novel, pharmacomechanical approach with prolonged infusion of urokinase in the occluded pulmonary arteries (PAs). Manual aspiration of thrombus using guide catheters was followed by introduction of thrombolysis catheters and a local bolus of urokinase. The lysis catheters were left in place, and repeat PA cine angiography and right-sided cardiac catheterization was performed 3 days later. A total of 63 patients (mean age 60 ± 15 years) were treated over 8 years: 17 patients (27%) had massive and 46 patients (73%) submassive PE. The mean PA pressure was 35 ± 10 mm Hg, and 54% had central bilateral PE. Five patients died, 1 before, 1 during, and 3 after the intervention. Nine patients (14%) had major bleeds (hemoglobin decrease >30 g/L), but in none of these patients was bleeding the reason for fatal outcome. After 3.3 ± 1.0 days, 49 of 58 living patients (84%) were restudied. In 29 (59%), there was a reduction of thrombotic burden by >90%, and in 14 (29%), the reduction was 50% to 90%. Mean PA pressure was reduced from 33 ± 8 to 21 ± 7 mm Hg (p <0.001), and this was not dependent on a reduction of thrombus. In conclusion, manual aspiration and application of prolonged thrombolysis is feasible and safe. Improvement of PA pressures is impressive and there is no correlation between morphologic disappearance of thrombus and normalization of PA pressures.
Pulmonary embolism (PE) is common and if left untreated is associated with high mortality. The use of catheter-based therapy has shown promising results in small studies, and catheter intervention can nowadays be considered an alternative to systemic thrombolysis or surgical embolectomy. It is not clear how catheter-based treatment is best delivered, and different approaches (thrombus fragmentation, rheolytic thrombectomy, suction thrombectomy, and rotational thrombectomy) have been suggested. The aim of this study was to assess a novel approach of pharmacomechanical treatment of massive and submassive PE. Besides describing the feasibility and safety of this new approach, we report how a reduction of thrombotic burden in the pulmonary arteries (PAs) affects hemodynamic measures in the subacute period of PE.
Methods
We studied a cohort of patients with massive and submassive PE treated between January 2004 and March 2012 with catheter-based therapies ( Figure 1 ) at the Luzerner Kantonsspital, Lucerne, Switzerland. Massive PE was defined as documentation of central or paracentral PE on computed tomography, systolic blood pressure <90 mm Hg, and evidence of 1 of the following: tissue hypoperfusion and hypoxia (saturation on air <92%), altered level of consciousness, oliguria, and cool or clammy extremities. Submassive PE was defined as documentation of central or paracentral PE on computed tomography, systolic blood pressure >90 mm Hg, right ventricular dilatation (right ventricular to left ventricular end-diastolic diameter on computed tomography >0.9 ) and hypoxia (saturation on air <92%) or elevation of cardiac necrosis markers, preferably troponin.
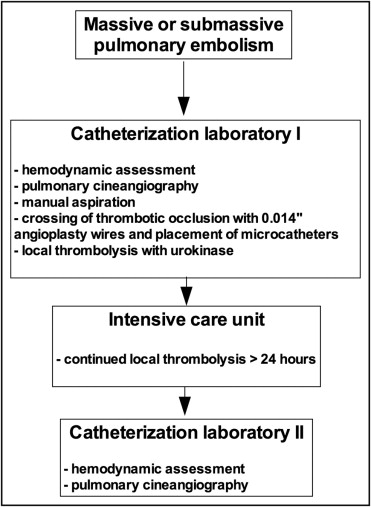
The intervention was performed in the catheterization laboratory, and the right femoral approach was preferred. Using a 6Fr multipurpose or pigtail catheter, the PA, right ventricular, and right atrial pressures were documented. Afterward, a 6Fr pigtail catheter was used for selective PA cine angiography (PCA) (25 ml at 12 ml/s) of the left and right PAs in the anteroposterior view. Once the occlusion was visualized, a 7Fr multipurpose or Amplatzer Left 2 catheter (St. Jude Medical, St. Paul, Minnesota) was used for manual aspiration using a 50-ml syringe. After manual aspiration, the thrombus was crossed using a 300-cm, 0.014-inch coronary angioplasty wire (Hi-Torque Balance Heavyweight; Abbott Vascular, Santa Clara, California). Thrombolysis microcatheters ( Figure 2 ) (Jet-Lysis catheter, 2.7Fr, 120 cm; Bard Angiomed, Karlsruhe, Germany) were placed in the occluded PA using the over-the-wire approach. Local thrombolysis was initiated by bolus injection of 125,000 U urokinase in each microcatheter. The catheters were left in place for ≥24 hours with continuous infusion of urokinase (250,000 U/6 h/catheter), and the patients were transferred to the intensive care unit. Fibrinogen was measured every 6 hours, and the infusion rate of urokinase was increased by 100,000 U/6 h/catheter if fibrinogen was >2.5 μmol/L and there was no bleeding. The goal was to achieve complete thrombolysis and normalization of PA pressures within 3 days ( Figure 3 ).

Major bleeding was considered a hemoglobin decrease of >30 g/L or the need for transfusion. Upon stabilization of clinical status, patients were brought back to the catheterization laboratory for catheter withdrawal, PCA, and hemodynamic assessment. After obtaining PA, right ventricular, and right atrial pressures, PCA was performed as described previously. Reduction of thrombotic burden was visually classified as moderate (<50% reduction), good (reduction of 50% to 90%), and excellent (>90% reduction).
Data are expressed as mean ± SD for continuous variables and absolute numbers with percentages for categorical variables. Comparisons between groups were performed using the unpaired Student’s t test. Hemodynamic values between the 2 time points were compared using a paired Student’s t test. A p value <0.05 was considered significant. The calculations were performed using GraphPad Prism version 5 for Mac (GraphPad Software, La Jolla, California). The procedures were considered clinically indicated, and patients consented to the intervention.
Results
The population included a total of 63 patients with a mean age of 50 ± 15 years. The characteristics of this population are summarized in Table 1 . PE was considered massive in 17 patients (27%) and submassive in 46 patients (73%). Systemic and right-sided cardiac pressures in patients with massive and submassive PE are summarized in Table 2 . Thirty-six patients (67%) had elevations of cardiac enzymes at presentation: mean troponin T was 0.19 ± 0.50 μg/L, and mean creatine kinase-MB was 6.5 ± 10.4 U/L. Procedural characteristics are summarized in Table 1 . Thrombolysis catheters were implanted in 58 patients (92%), and in 4 patients (6%) aspiration only was performed. A total of 5 patients (8%) died. One patient (2%) died before treatment could be started. One patient had a spontaneous lung bleed before the intervention and died during the intervention. Three patients died after the intervention, at 2, 24, and 48 hours after thrombus aspiration and introduction of thrombolysis catheters. Repeat right-sided cardiac catheterization and PCA were performed in 49 survivors (84%). Hemodynamic comparisons between baseline values and repeat measurements are presented in Table 3 .
| Variable | Value |
|---|---|
| Age (years) | 60 ± 15 |
| Men | 37 (59%) |
| Clinical setting | |
| Dyspnea | 50 (79%) |
| Syncope | 4 (6%) |
| Chest pain | 3 (5%) |
| Cardiopulmonary resuscitation | 3 (5%) |
| Unspecific symptoms | 3 (5%) |
| Massive PE | 17 (27%) |
| Submassive PE | 46 (73%) |
| Localization of PE | |
| Central bilateral | 34 (54%) |
| Central single side | 6 (10%) |
| Paracentral | 23 (36%) |
| Systolic blood pressure (mm Hg) | 116 ± 29 |
| Diastolic blood pressure (mm Hg) | 70 ± 18 |
| Heart rate (beats/min) | 99 ± 2 |
| PA systolic pressure (mm Hg) | 55 ± 16 |
| PA diastolic pressure (mm Hg) | 21 ± 8 |
| PA mean pressure (mm Hg) | 35 ± 10 |
| Right ventricular systolic pressure (mm Hg) | 55 ± 17 |
| Right ventricular diastolic pressure (mm Hg) | 15 ± 7 |
| Mean right atrial pressure (mm Hg) | 13 ± 6 |
| Procedural characteristics | |
| Screening time (minutes) | 14.0 ± 7.2 |
| Contrast use (ml) | 132 ± 62 |
| Aspiration only | 4 (6%) |
| Thrombolysis catheters | 58 (92%) |
| Number of catheters | 2.8 ± 1.2 |
| Vena cava filter implantation | 5 (8%) |
| Duration of thrombolysis (days) | 3.3 ± 1.0 |
| Variable | Massive PE | Submassive PE | p Value |
|---|---|---|---|
| Systemic | |||
| Systolic blood pressure (mm Hg) | 80 ± 10 | 129 ± 21 | <0.001 |
| Diastolic blood pressure (mm Hg) | 48 ± 10 | 78 ± 13 | <0.001 |
| Heart rate (beats/min) | 114 ± 18 | 93 ± 18 | <0.001 |
| PA pressure | |||
| Systolic (mm Hg) | 57 ± 15 | 54 ± 17 | 0.53 |
| Diastolic (mm Hg) | 24 ± 10 | 21 ± 7 | 0.20 |
| Mean (mm Hg) | 37 ± 11 | 34 ± 10 | 0.24 |
| Right ventricular pressure | |||
| Systolic (mm Hg) | 58 ± 17 | 54 ± 17 | 0.36 |
| Diastolic (mm Hg) | 17 ± 4 | 14 ± 7 | 0.13 |
| Right atrial pressure (mm Hg) | 17 ± 7 | 12 ± 6 | 0.01 |



