Use of Radionuclide Imaging in Acute Coronary Syndrome
Seth Uretsky
Edgar Argulian
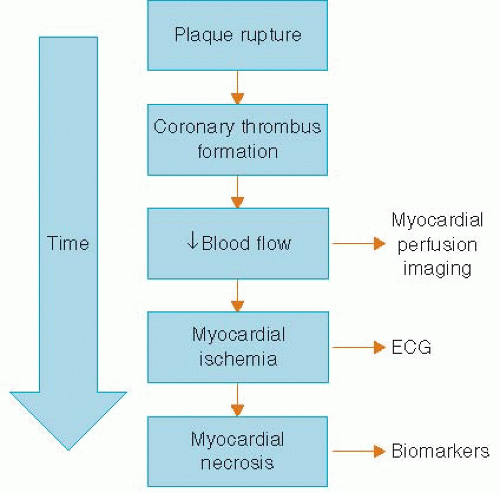
Chest pain is a common complaint and is often the presenting symptom in patients with acute coronary syndrome (ACS).1 The evaluation of chest pain as a presenting symptom for ACS is complicated by (1) the numerous etiologies that can cause chest pain, (2) the lack of a highly specific and sensitive test available at the time of initial evaluation, (3) poor clinical outcomes of patients in whom a diagnosis of ACS is missed, and (4) the medicolegal implications. Because patients with ACS are at a high risk for poor outcomes, the ability to identify these patients rapidly during the initial evaluation is critical. The initial diagnostic workup in patients presenting with symptoms suspicious of ACS include a history, physical examination, ECG, and serum troponin. The ECG is the key diagnostic test used to determine whether the patient has ongoing myocardial ischemia. Unfortunately, the ECG is often nondiagnostic in many patients who present with symptoms suspicious for ACS. Although serum troponin has been shown to be a sensitive test for myocardial damage, there are certain drawbacks to the test (i.e., the need for ischemia of sufficient time for myocardial necrosis to take place), and the initial serum troponin is often negative in patients with ACS. Based on the pathophysiology of coronary artery plaque rupture as the cause of ACS, one of the earliest signs of ACS is the decrease in blood flow to the myocardium (Figure 6.1). This decrease in myocardial blood flow can be evaluated using single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI), which images the relative amount of myocardial blood flow to all segments of the left ventricle. The use of SPECT MPI in the patient presenting with chest pain was developed over the last several decades to allow for rapid triage in chest pain patients with an eye towards initiation of the necessary medical therapy and revascularization procedures (primary angioplasty or thrombolysis) as quickly as possible, thereby reducing the amount of myocardial necrosis. However, we would also like to identify those patients in whom the chest pain symptoms are not because of ACS so that they are not unnecessarily admitted to the hospital, thus better utilizing health care resources. In this chapter, we will explore the technical, physiologic, and current outcomes data of using SPECT MPI in patients with acute chest pain.
SPECT MPI OVERVIEW
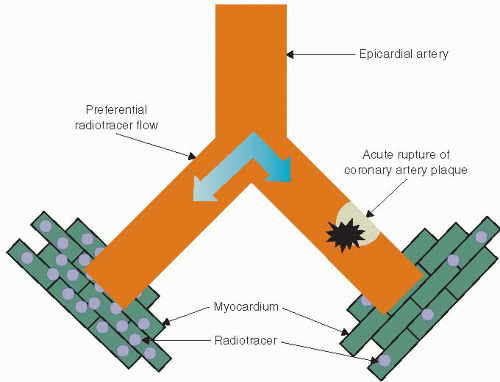
MPI was introduced in the 1970s into clinical practice as a tool to noninvasively assess for myocardial ischemia. In the 1980s planar imaging gave way to the more robust SPECT imaging most commonly used today. The theory behind SPECT MPI relies on the fact that blood flows preferentially down the path of least resistance, that is, the vessel without intracoronary thrombus versus the vessel with intracoronary thrombus (Figure 6.2). When comparing myocardial regions with each other, a decrease in tracer uptake in a region is a sign of myocardial hypoperfusion. SPECT MPI utilizes radiotracers whose activity can be detected with a special camera called a scintillation camera. The most commonly used radiotracers are thallium-201 (Tl-201) and technetium-99m (Tc-99m). Radiotracers contain atoms that undergo radioactive decay by emitting a particle that can be detected using a SPECT camera. Once injected intravenously, the radiotracer is taken up by the myocardium and the relative “amount” of tracer in each region of the myocardium can be measured and imaged.
SPECT CAMERA
The SPECT camera is designed to detect the radioactivity emitted from a patient after injection of a radiotracer. After injection of the radiotracer, patients are placed in the camera and the images are acquired.
RADIOTRACERS
Radiotracers are tagged molecules that emit radioactivity. The ideal radiotracer has several features:
Linear relationship between myocardial uptake and blood flow
High myocardial extraction rate
Low extra-cardiac uptake and
Does not decay or redistribute too rapidly.
The most commonly used radiotracers in nuclear cardiology are Tl-201 and Tc-99m. Tc-99m has several advantages over Tl-201, including a shorter half-life, higher-energy photon, poor redistribution, and it is available locally through an Mo-99 generator. The process whereby Thallium decays is called electron capture or isomeric transition (Tl-201 + e− → Hg-201 + γ), whereas Tc-99m decays by the process called gamma decay (Tc-99m → Tc-99 + γ).
MYOCARDIAL PERFUSION
MPI imaging is based on the concept that the injected radiotracer is taken up by the myocardium in proportion to the amount of blood flow that myocardial region receives. If blood flow to an area of myocardium is reduced owing to an intracoronary thrombus, this area of myocardium will receive less radiotracer in comparison with areas of myocardium in which blood flow is not reduced (Figure 6.2). The resulting SPECT images will have a decrease in tracer activity in the myocardial segments subtended by the diseased coronary artery. In patients with chest pain owing to acute coronary insufficiency, there is rupture of coronary plaque resulting in the formation of intracoronary thrombus and a subsequent decrease in myocardial blood flow to the myocardium supplied by that artery. However, in patients without the presence of intracoronary thrombus, there will be no decrease in blood flow to the myocardium, and no decrease in tracer activity will be seen.
SPECT MPI INTERPRETATION
By convention SPECT images are displayed in three orientations: short axis, vertical long axis, and horizontal long axis (Figure 6.3). The left ventricle can be divided into the anterior wall, lateral wall, inferior wall, and septum. SPECT images are displayed with the post-stress images on the top line and the rest images below (Figure 6.4). The short-axis images are displayed apex to base, followed by the vertical long axis, and the horizontal long axis. As discussed above, SPECT MPI is measuring relative blood flow to the various segments of the myocardium. When interpreting SPECT images, one compares the amount of tracer uptake on the stress images with the amount of tracer uptake in the rest images for each myocardial segment. If the tracer uptake is homogeneous in both the rest and stress images, there is no decrease in blood flow to the myocardium, that is, no ischemia or scar (Figure 6.4




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree