The prevalence of valvular heart disease is strongly influenced by the patient’s age. In the United States and other developed countries, significant valvular disease tends to cluster at age extremes; in the young it results primarily from congenital heart disease (CHD) and in the elderly from degenerative wear-and-tear valve changes.
The combined prevalence of moderate and severe CHD is approximately 5 in 1,000 live births.
1 The prevalence of congenital valvular disease is much lower as the bulk of the CHD is accounted for by nonvalvular lesions such as ventricular and atrial septal defects, and patent ductus arteriosus. The prevalence of valvular disease among the elderly ≥75 years of age is approximately 13.3%. This is in contrast to the prevalence rate of 0.7% in the 18- to 44-years age group.
2 Between the age extremes, valvular disease is encountered mostly in the survivors of CHD or in immigrants with rheumatic valvular disease.
Thus a patient in an adult coronary care unit (CCU) in the United States usually falls into one of the following three categories: elderly patients, patients with CHD, and immigrants with rheumatic heart disease. The last two groups often include pregnant women.
Valvular disease presents either as valvular stenosis or valvular regurgitation. When valvular disease is the primary reason for a CCU admission, the patient usually presents with severe degrees of stenosis, regurgitation, or a combination thereof. Among the elderly CCU patients typical valvular disorders include aortic stenosis (AS) and mitral regurgitation; in addition, there is often secondary tricuspid regurgitation. In younger CCU patients, typical valvular disorders include rheumatic mitral stenosis, tricuspid valve endocarditis, and severe pulmonic insufficiency arising as a long-term complication of CHD surgeries performed in childhood.
In general, medical therapy of severe valvular disease is limited to symptom relief and hemodynamic stabilization in preparation for definitive treatment performed by interventional cardiologists and cardiac surgeons. Unfortunately, there is a paucity of solid randomized trial data to support any form of treatment of valvular disease.
3 In the latest joint guidelines of the American Heart Association and the American College of Cardiology, most of the recommendations for the management of valvular disease are based on either nonrandomized trials or expert opinions.
4
ANTIBIOTIC PROPHYLAXIS OF VALVULAR HEART DISEASE
Routine antibiotic prophylaxis in no longer recommended for acquired valvular disease in a nontransplanted heart unless there is a prior history of endocarditis. For congenital forms of valvular disease in the setting of surgically repaired CHD, antibiotic prophylaxis is recommended only if (1) there is prosthetic material or device, whether placed by surgery or by catheter intervention, during the first 6 months after the procedure, and (2) there are residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (which inhibit endothelialization).
5
AORTIC STENOSIS
ETIOLOGY AND PATHOPHYSIOLOGY
AS in a typical adult CCU patient arises from calcific degeneration of either a previously normal trileaflet aortic valve (TAV) or a congenitally bicuspid aortic valve (BAV). Calcification process is often enhanced by disorders of perturbed calcium phosphate metabolism such as end-stage renal disease and Paget’s disease.
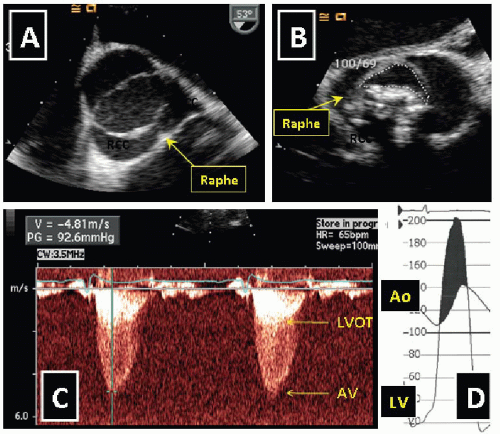
6Calcific TAV stenosis is typically encountered among the elderly and calcific BAV stenosis in middle-aged patients (
Figure 25.1). It is estimated that AS is present in 2% of all Americans, 65 years of age or older.
2 TAV stenosis may be a manifestation of generalized atherosclerosis. BAV is the commonest congenital heart defect occurring in approximately 1% to 2% of all live births.
7 BAV may present as stenosis or regurgitation and may be associated with aortopathies such as aortic aneurysm, aortic dissection, and coarctation.
8The major cardiovascular adaptation to pressure overload caused by AS is left ventricular hypertrophy (LVH). Because of LVH the left ventricular chamber becomes smaller and its wall thicker; both these changes lower the wall stress and allow for preservation of left ventricular ejection fraction (LVEF) for very long periods. When LVEF starts to decrease it is often due to an afterload-LVH mismatch (not enough LVH for the degree of AS) rather than cardiomyopathy.
CLINICAL PRESENTATION
AS is usually asymptomatic unless severe. Patients with severe AS typically present with angina, syncope, sudden death, or heart failure. Physical diagnosis may establish the diagnosis of AS but is often incapable of precisely grading AS. Classic auscultatory findings of AS include a systolic crescendo-decrescendo ejection murmur over the precordium that radiates to the neck, and an occasional absence of the valve closing S2 sound.
9 Carotid upstroke is often weak and delayed (pulsus parvus & tardus).
MEDICAL THERAPY
There are no proven medical therapies for prevention or treatment of AS. Antibiotic prophylaxis of bacterial endocarditis in patients with AS is no longer recommended; for details see section on Antibiotic Prophylaxis of Valvular Heart Disease in this chapter’s introduction.
Patients with severe AS often present to CCU with clinical signs of heart failure. Diuretics, angiotensin converting enzyme (ACE) inhibitors, and digitalis may be used with caution. Although historically the use of intravenous vasodilators was contraindicated in patients with severe AS, a small study in CCU patients have demonstrated that intravenous nitroprusside actually relieves symptoms of heart failure in patients with severe AS and severely reduced left ventricular systolic function.
11 In that study, intravenous nitroprusside was started at a mean dose of 14 ± 10
µg per minute, and the dose was increased to a mean of 103 ± 67
µg per minute at 6 hours and 128 ± 96
µg per minute at 24 hours.
SURGICAL AND PERCUTANEOUS INTERVENTIONAL THERAPY
AS is a mechanical problem that requires a mechanical solution. Surgical aortic valve replacement (AVR) is currently the preferred therapeutic choice in patients with AS as it improves symptoms and survival. Major indications for AVR are listed in
Table 25.3.
Percutaneous interventions for AS include aortic valve balloon valvuloplasty and percutaneous AV replacement. Balloon valvuloplasty is an effective form of therapy for congenital AS. However, balloon valvuloplasty in calcific TAV or BAV aortic stenosis has been rather ineffective (valve area seldom increases above 1.0 cm2 and there is a high rate of restenosis), and it has significant morbidity and mortality. At present, typical indications for balloon valvuloplasty in calcific AS include temporary relief of AS in patient undergoing noncardiac surgery (such as hip replacement) or in those with terminal illness (such as cancer).
Percutaneous valve replacement is a new and promising therapy for AS. Although the first percutaneous implantation of an aortic prosthetic valve has been reported in 2002, such a procedure in the United States is gradually moving from an investigational use within clinical trials to clinical practice.
12
IMPACT ON PREGNANCY
Patients with calcific AS are seldom of child-bearing age. In patients of child-bearing age, AS is usually caused by noncalcific congenital abnormalities (such as unicuspid aortic valve). Young patients with moderate-to-severe AS should be advised against conception until AS is relieved. Those who nonetheless become pregnant may or may not develop severe symptoms. Pregnant patients with AS and mild symptoms may be managed conservatively during pregnancy with bed rest, oxygen, and β-blockers. Pregnant patients with severe symptoms may need percutaneous or even surgical intervention. These procedures carry significant risk to both the mother and the unborn child.
AORTIC REGURGITATION
ETIOLOGY AND PATHOPHYSIOLOGY
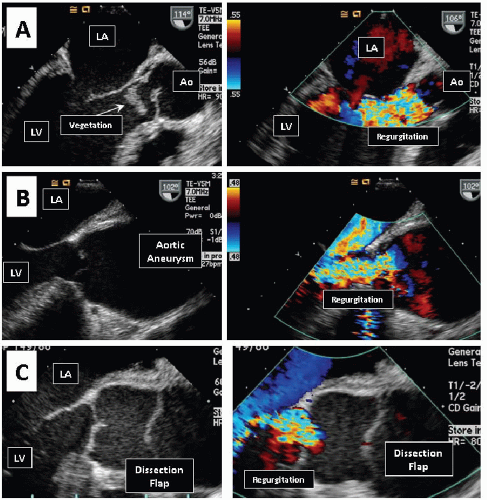
Aortic regurgitation (AR) may be due to disorders of the aortic root or the aortic valve leaflets. BAV, ascending aortic aneurysm, aortic dissection, and endocarditis are common
causes of AR. Conditions that predispose patient to aortopathies and AR include systemic hypertension and connective tissue disorders (such as Marfan syndrome, Ehlers-Danlos syndrome, and ankylosing spondylitis). In the less developed countries, rheumatic heart disease and tertiary syphilis remain important causes of AR.
In AR, a regurgitant volume flows back into the ventricle through the aortic valve during diastole. There it joins the systemic volume that had entered the left ventricle through the mitral valve. During subsequent systole the two volumes leave the aortic valve together; the systemic volume continues into the aorta and its branches, whereas the regurgitant volume flows back into the ventricle. The cycle then repeats itself. Subsequent pathophysiology depends on whether AR is chronic or acute.
In chronic AR, there is a progressive enlargement of the left ventricle to accommodate the combined systemic stroke volume and the regurgitant volume. This remodeling often prevents significant elevation of left heart pressures. It may take years if not decades for patients with chronic AR to develop congestive heart failure owing to progressive left ventricular systolic dysfunction.
This is in sharp contrast to acute AR where there is sudden volume overload of a nondilated left ventricle, marked elevation of left ventricular and left atrial pressures, life-threatening pulmonary edema, and even cardiogenic shock.