FIGURE 20.1. Virchow’s triad of thrombus formation.
First described by Paget and von Schrötter in the late 1800s and subsequently named the “Paget-Schrötter syndrome,” a significant fraction of cases of primary upper extremity DVT are caused by compression of the axillosubclavian venous segment at the thoracic outlet, usually in the vicinity of the first rib. This is commonly referred to as “venous thoracic outlet syndrome.” It is hypothesized that thrombosis is preceded by many years of intermittent compression and repetitive trauma to this venous segment, accompanied by the accumulation of scar tissue. In these patients, compression of the subclavian vein can be induced during the performance of upper extremity venography by several provocative body positions, including hyperabduction and external rotation of the shoulder, extension of the neck, and caudal and posterior movement of the shoulder.5 However, the situation is complicated by the fact that many asymptomatic individuals will also demonstrate these changes on provocative venography. Additionally, a minority of patients with primary upper extremity DVT, many of whom have hypercoagulable states, have no evidence of thoracic outlet compression even with provocative arm and shoulder positioning.
Approximately 75% of upper extremity DVT cases are termed “secondary” DVT, the majority of which are caused by the presence of indwelling medical devices such as central venous catheters and pacemaker wires.2,6 In addition, the presence of known or occult malignancies, with or without an indwelling catheter, is an important factor in the pathogenesis of secondary upper extremity DVT.7 In hospital settings, secondary DVT, particularly those cases associated with indwelling catheters and pacemakers, is far more common than is primary upper extremity DVT.
Diagnosis
The majority of patients presenting with the Paget-Schrötter syndrome (also called “effort thrombosis”) are young, athletic, and often muscular males, although the condition is certainly not limited to this demographic group. The clinical hallmark of the Paget-Schrötter syndrome is ipsilateral upper extremity swelling that typically involves the upper arm, forearm, and hand and is predominantly nonpitting, particularly during the acute phase. Pain is also a frequent symptom, usually described as “tightness” or “heaviness,” and is often exacerbated by dependency and partially ameliorated by elevation. Because these patients have venous hypertension in the affected extremity, the veins of the arm and hand are often distended, although this may not be readily apparent because the venous distention is often masked by edema. Although not necessarily present in the acute phase, enlarged venous collaterals are usually visible around the shoulder.
The clinical presentation of patients with catheter-associated upper extremity DVT can be more subtle than the presentation of Paget-Schrötter syndrome.8 Thrombus associated with indwelling catheters often develops more slowly (allowing more time for the development of venous collaterals) and typically involves a shorter venous segment than primary DVT. Furthermore, the affected patients usually have multiple medical comorbidities (e.g., cancer, heart failure, end-stage renal disease) rendering them far more sedentary than the typical patient with Paget-Schrötter syndrome.
Prior to obtaining objective diagnostic tests, it is possible to stratify patients according to low, intermediate, and high pretest probability for upper extremity DVT in much the same way as has been demonstrated for lower extremity DVT. Constans et al9 used logistic regression on a derivation sample of 140 patients. These authors developed a clinical scoring system in which a point is added for the presence or absence of swelling, pain, and an indwelling catheter or pacemaker, and a point is subtracted when there is a plausible alternative diagnosis. The scoring system was then tested in a 214 patient validation sample. Among patients with a score of −1 or 0 (low probability), 1 (intermediate probability), and 2 or 3 (high probability), upper extremity DVT was found in 13%, 38%, and 69% of patients, respectively.
Because of its low specificity, a positive d-dimer test cannot be used to diagnose upper extremity DVT. A negative d-dimer result can be used to confidently rule out DVT, particularly among patients with a low pretest probability of upper extremity DVT. However, the clinical usefulness of this approach is limited by the fact that the majority of patients with suspected upper extremity DVT who have cancer or indwelling catheters or pacemakers will also have elevated d-dimer results.10
The most commonly employed diagnostic imaging tests are duplex ultrasonography and catheter venography, although magnetic resonance venography (MRV) and computed tomography venography (CTV) may also play important roles depending on local expertise and availability. Although studies of MRV show particularly promising results when performed in centers of excellence, this discussion focuses on duplex scanning because it is far more commonly used in clinical practice, is less costly, and does not involve the use of contrast agents that can pose risks to the patient.
Table 20.1 summarizes the studies in which the accuracy of duplex scanning for the diagnosis of upper extremity DVT has been quantified using catheter contrast venography as the reference or “gold standard.”6,11–16 However, it should be emphasized that venography is not a perfect standard in this setting. When two “blinded” radiologists interpret the same venogram, there is considerable disagreement.17 In addition, there are cases of nonocclusive upper extremity DVT diagnosed by duplex scanning in which the venogram is interpreted as being “normal,” and venography is clearly inferior to duplex scanning in detecting jugular venous thrombosis. These caveats aside, a well-performed venogram is, in most clinical settings, the final arbiter for the presence or absence of upper extremity DVT.
TABLE 20.1 Summary of Reported Studies in which the Results of Duplex Scanning for Axillary and Subclavian Venous Thrombosis Were Validated Using Venography as the “Gold Standard”
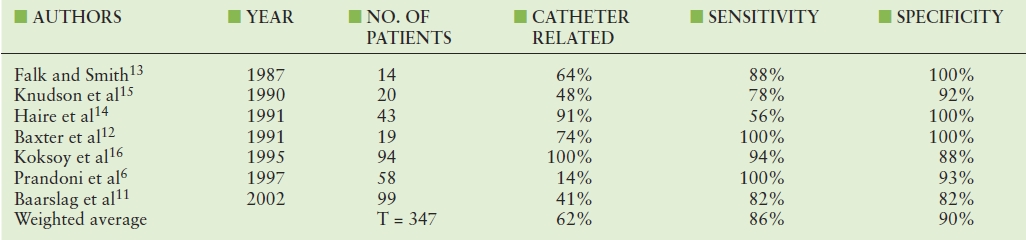
The accuracy of duplex scanning in diagnosing DVT of the upper extremities has been far less extensively studied than in the lower extremities. The published studies (see Table 20.1) have demonstrated considerable variability in study methodologies, including the proportion of patients with catheter-associated DVT. Taking a weighted average of these studies yields a sensitivity (86%) that is slightly lower than the specificity (90%), but the heterogeneity in reported results is striking, ranging from 56% to 100% for sensitivity and from 82% to 100% for specificity. Some of the variability in results is almost certainly due to the fact that duplex scanning is operator dependent, and this operator dependence is probably more of a factor in the upper extremity venous system owing to the difficulty of imaging underneath the clavicle where most clinically important upper extremity DVT occurs. A list of indications for upper extremity venous duplex scanning is given in Table 20.2.
TABLE 20.2 Indications for Upper Extremity Venous Duplex Scanning

PICC, peripherally inserted central catheter; SVC, superior vena cava.
While upper extremity venous duplex scanning is the most frequently employed imaging test for the diagnosis of DVT in most centers, there is still an important subset of patients who are appropriate for venography. Among patients for whom there is an intermediate or high clinical suspicion of upper extremity DVT, particularly those who would be candidates for catheter-directed thrombolysis, a strong argument can be made for ignoring a negative duplex scan result or omitting the duplex scan altogether and proceeding directly to venography. Conversely, for patients who are not considered candidates for catheter-directed thrombolysis, the decision to anticoagulate is often based solely on the results of the duplex scan. Among patients who are not considered candidates for thrombolysis and who have had a negative duplex scan, but for whom there is still a high clinical suspicion of upper extremity DVT, venography should be considered and MRV or CTV may also be useful. It should be noted that no studies have examined clinical outcomes resulting from withholding treatment on the basis of a negative duplex scan of the upper extremity veins.
Prognosis
Since the majority of patients with upper extremity DVT are treated with anticoagulation, little is known about the natural history of upper extremity DVT without treatment. Clinically important outcomes include mortality, PE, recurrent thrombosis, and alleviation of acute and chronic (“postthrombotic syndrome”) symptoms. In most modern series of upper extremity DVT, mortality rates are substantial owing to the coexistence of cancer, central venous catheters, cardiac disease, pacemakers, and automatic implantable cardiac defibrillators.3,18–20 The vast majority of these deaths are due to the patient’s underlying disease rather than to PE. In contrast, patients with Paget-Schrötter syndrome are typically young and healthy with a markedly better prognosis.
As mentioned previously, PE, and particularly symptomatic PE, is less frequently associated with upper extremity DVT than with lower extremity DVT. Explanations for this difference include the smaller size of upper extremity venous thrombi and variations in mechanical forces and flow dynamics. In studies with heterogeneous combinations of primary and secondary DVT and treatment strategies ranging from no anticoagulation to standard anticoagulation with or without thrombolytic therapy, the incidence of reported PE associated with upper extremity DVT has ranged from 0% to 25%.4,21–26 Because the majority of emboli associated with DVT of the brachial and cephalic veins are asymptomatic, the frequency of detected PE will depend on how aggressively the diagnosis is sought. In a prospective study of 86 patients with catheter-associated upper extremity DVT, all of whom were anticoagulated and received ventilation-perfusion lung scans, Monreal et al25 found PE in 13 patients (15%); 11 of these patients were asymptomatic, while 2 had symptomatic and fatal PE.
In a comprehensive literature review of patients with primary upper extremity DVT, Thomas and Zierler26 found a reported incidence for PE of 12% among those treated without anticoagulation (rest, heat, and elevation of the effected limb) versus 7% for those who were anticoagulated. Although not known with certainty, the rate of PE is most likely similar among patients with primary versus secondary upper extremity DVT. While the data on jugular vein thrombosis are sparse, the available literature suggests a similar risk of PE compared with that of upper extremity DVT.21,24,27 It is generally believed that isolated DVT occurring distal to the shoulder (i.e., in the brachial or forearm veins), as well as superficial thrombophlebitis involving the cephalic and basilic veins, are both associated with very low risks of PE. However, PE has been reported in both of these subgroups, including one study of 52 patients with isolated brachial vein thrombosis in which the proportion of patients with documented PE was 11.5%.28
The risks of recurrent thrombosis and chronic postthrombotic symptoms are also substantially lower following upper extremity DVT compared with lower extremity DVT. The risk of symptomatic upper extremity recurrent thrombosis is 2% to 5% at 12 months and 5% to 15% at 5 years.3,18,29 The corresponding figures for lower extremity DVT are 5% to 15% at 12 months and 20% to 30% at 5 years.30,31 As previously mentioned, the risk of long-term postthrombotic symptoms is considerably lower in the arm than in the leg. The proportion of patients suffering long-term postthrombotic symptoms following upper extremity DVT is approximately 15% compared to 30% to 60% following lower extremity DVT.31–33 Patients who suffer long-term postthrombotic symptoms in the upper extremity are more likely to have residual axillosubclavian vein occlusion and are less likely to have suffered catheter-associated thrombosis.32
Treatment
The goals of treatment for patients with upper extremity DVT are to prolong life, alleviate acute symptoms, and minimize the risk of PE, symptomatic recurrent thrombosis, and the postthrombotic syndrome. The treatment of upper extremity DVT may consist of one or more of the following elements: conservative management (rest, heat, elevation, external compression), anticoagulation, central venous catheter removal, thrombolytic therapy, and surgical decompression of the thoracic outlet with or without open or endovascular venous reconstruction.
Because randomized trials of anticoagulant therapy in patients with upper extremity DVT are lacking, recommendations regarding the choice of anticoagulant and duration of therapy for patients with upper extremity DVT are largely extrapolated from data on the treatment of lower extremity DVT. Consensus guidelines from the American College of Chest Physicians provide detailed recommendations for the treatment of upper extremity DVT.34 Salient points from the most recent 9th edition of this guideline statement are reviewed here.
Unless there are contraindications, patients with acute thrombosis of the subclavian or axillary veins should be anticoagulated.34 Absolute contraindications to anticoagulation include severe active bleeding, intracranial bleeding, recent brain, eye, or spinal cord surgery, pregnancy, and malignant hypertension. Relative contraindications include recent stroke or major surgery and severe thrombocytopenia. In general, superficial venous thrombophlebitis of the cephalic or basilic veins and isolated DVT involving the brachial or forearm veins do not require anticoagulation. However, there is recent evidence to suggest that patients with superficial venous thrombosis of the lower extremity benefit from treatment with prophylactic dose anticoagulation.35 Although somewhat controversial, anticoagulation should be considered in patients with extensive and proximal superficial thrombophlebitis or brachial vein thrombosis when there is active cancer and/or indwelling catheters.
Anticoagulant therapy is typically started parenterally in the form of intravenous unfractionated heparin (IVUFH) or subcutaneous low molecular weight heparin (LMWH). Data from several randomized controlled trials and meta-analyses suggest that LMWH is superior to IVUFH with lower mortality and lower risk of recurrent thrombosis and major bleeding.19,36 Although less compelling, there is evidence to suggest that fondaparinux is also superior to IVUFH for patients with acute DVT and should be considered to have equivalent efficacy and safety compared with LMWH.34 Because LMWH and fondaparinux have significant renal excretion, they are contraindicated in patients with significant renal impairment (glomerular filtration rate <30 mL/min).
After parenteral therapy is begun, most patients with upper extremity DVT should be treated with a 3-month course of vitamin K antagonists.34 Selected patients with unprovoked DVT, thrombophilia, or cancer who are at low risk of bleeding complications may benefit from a longer course of therapy. There is evidence from lower extremity DVT trials suggesting that LMWH is superior to vitamin K antagonists for patients with active cancer.34
Patients with catheter-associated upper extremity DVT should be anticoagulated similarly. If the catheter is still needed, it should not be removed. If the catheter is no longer needed or is nonfunctional, it should be removed followed by a 3-month course of anticoagulant therapy as outlined above. Anticoagulant prophylaxis for patients with central venous catheters is controversial, but there is no convincing evidence to support it. A meta-analysis of seven trials showed a nonsignificant reduction in the risk of symptomatic thrombosis associated with thromboprophylaxis.37 This is an area requiring further study.
The use of superior vena cava (SVC) filters is another controversial topic. Major complications such as cardiac tamponade and aortic perforation have been reported in small observational studies.38 SVC filters should be limited to patients who have a contraindication to anticoagulation and those who have severe symptoms, and they should preferably only be placed in centers with experience using this technique.
Thrombolytic therapy should be limited to patients with severe symptoms, a short (<14 days) duration of symptoms, good functional capacity, and a low risk of bleeding. The majority of patients with secondary upper extremity DVT are not good candidates for thrombolytic therapy for several reasons. First, the symptoms are typically not severe. Second, the patients are often debilitated with significant comorbidities, and the risks of thrombolysis and surgery may be significant. Third, these patients tend to have acceptable results with anticoagulation alone.
In contrast, patients presenting with primary upper extremity DVT are often good candidates for thrombolytic therapy. The rationale for this statement lies in the observation that a significant proportion of patients with primary upper extremity DVT who are treated with anticoagulation alone may have persistent and often severe postthrombotic symptoms in the involved extremity.39 As previously stated, a significant fraction of patients with primary upper extremity DVT are young and active, and many are athletes. With vigorous exercise, the venous outflow from an upper extremity with a collateralized central venous occlusion often cannot keep up with arterial inflow. These patients have persistent arm swelling and pain exacerbated by extremity exercise. Because these patients are usually quite healthy, there are rarely any contraindications to thrombolytic or surgical therapy. Catheter-directed thrombolytic therapy is performed by embedding a multi–side-holed catheter into the thrombus at the time of venography and infusing a thrombolytic agent, usually tissue-type plasminogen activator (t-PA), typically for 12 to 24 hours. Catheter-directed thrombolysis is often combined with mechanical techniques (“pharmacomechanical thrombolysis”) that involve fragmentation and aspiration of the thrombus. These catheter-based approaches are more effective, less damaging to the endothelium, and less morbid than surgical thrombectomy (Fig. 20.2).
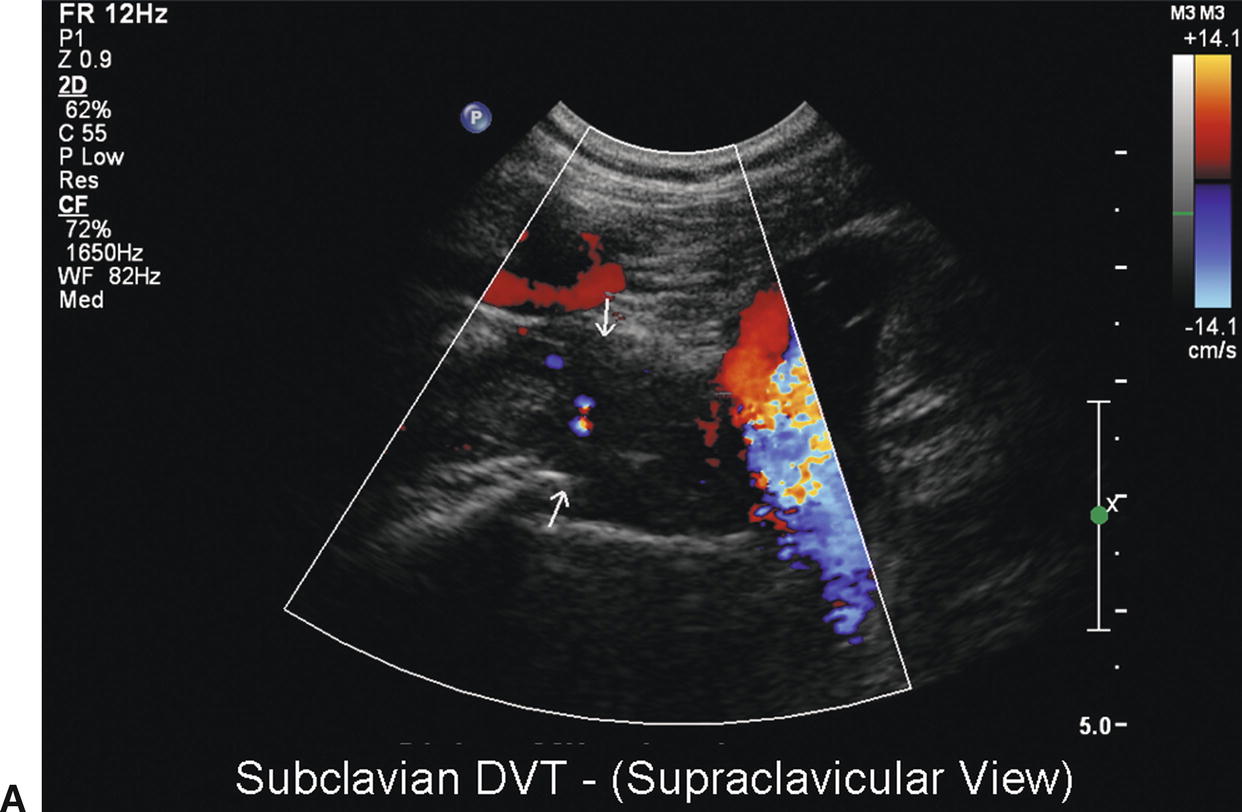
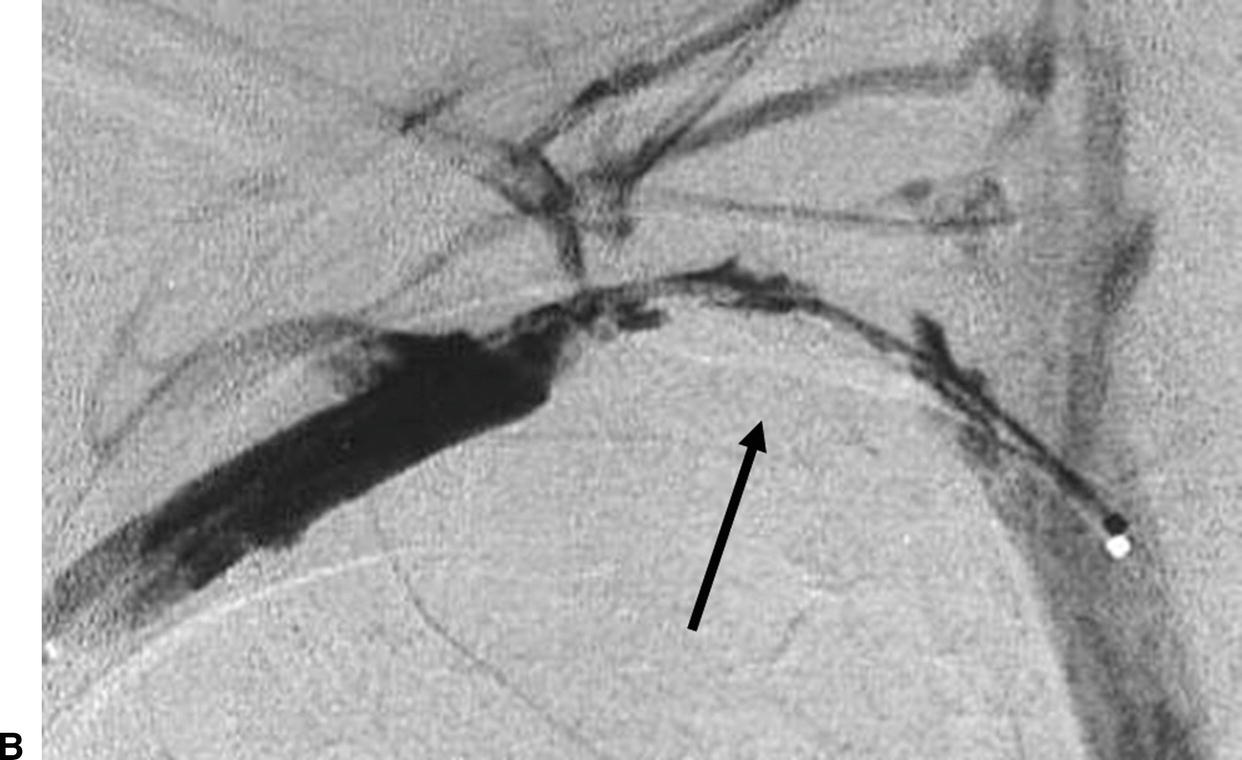
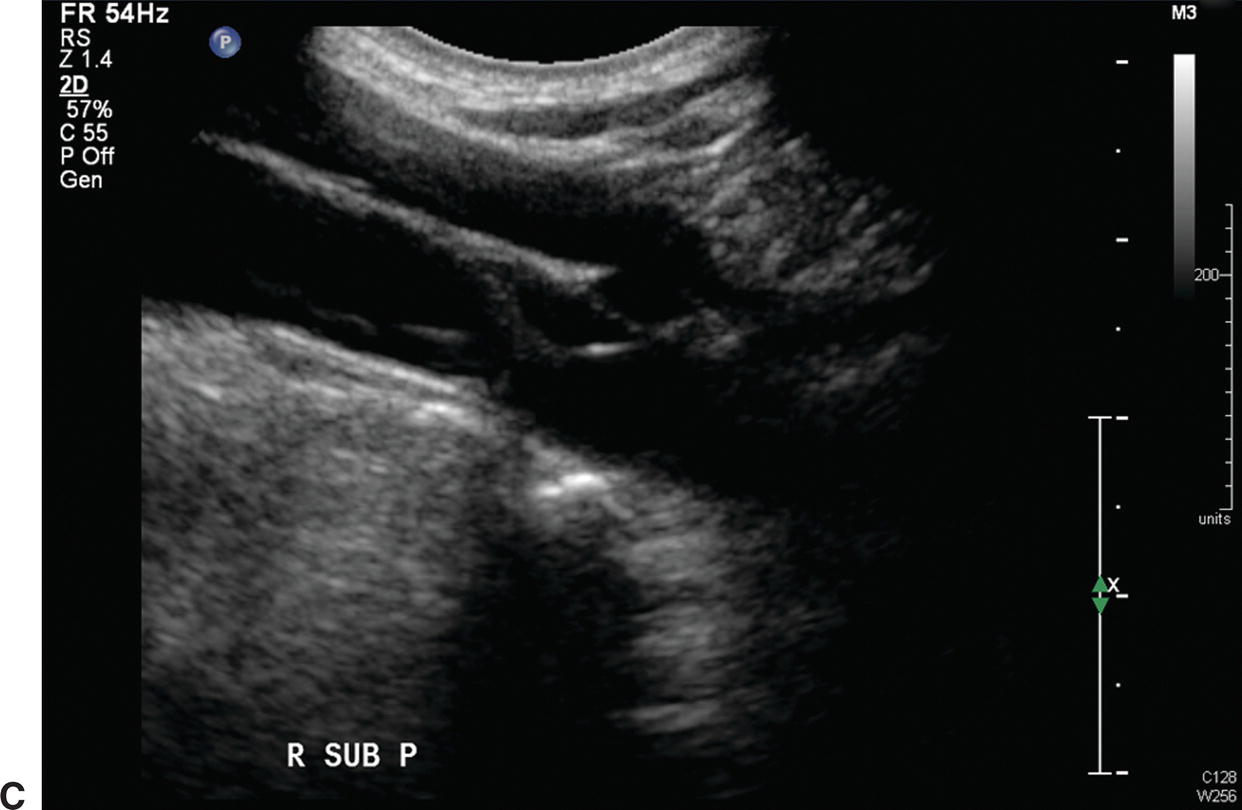
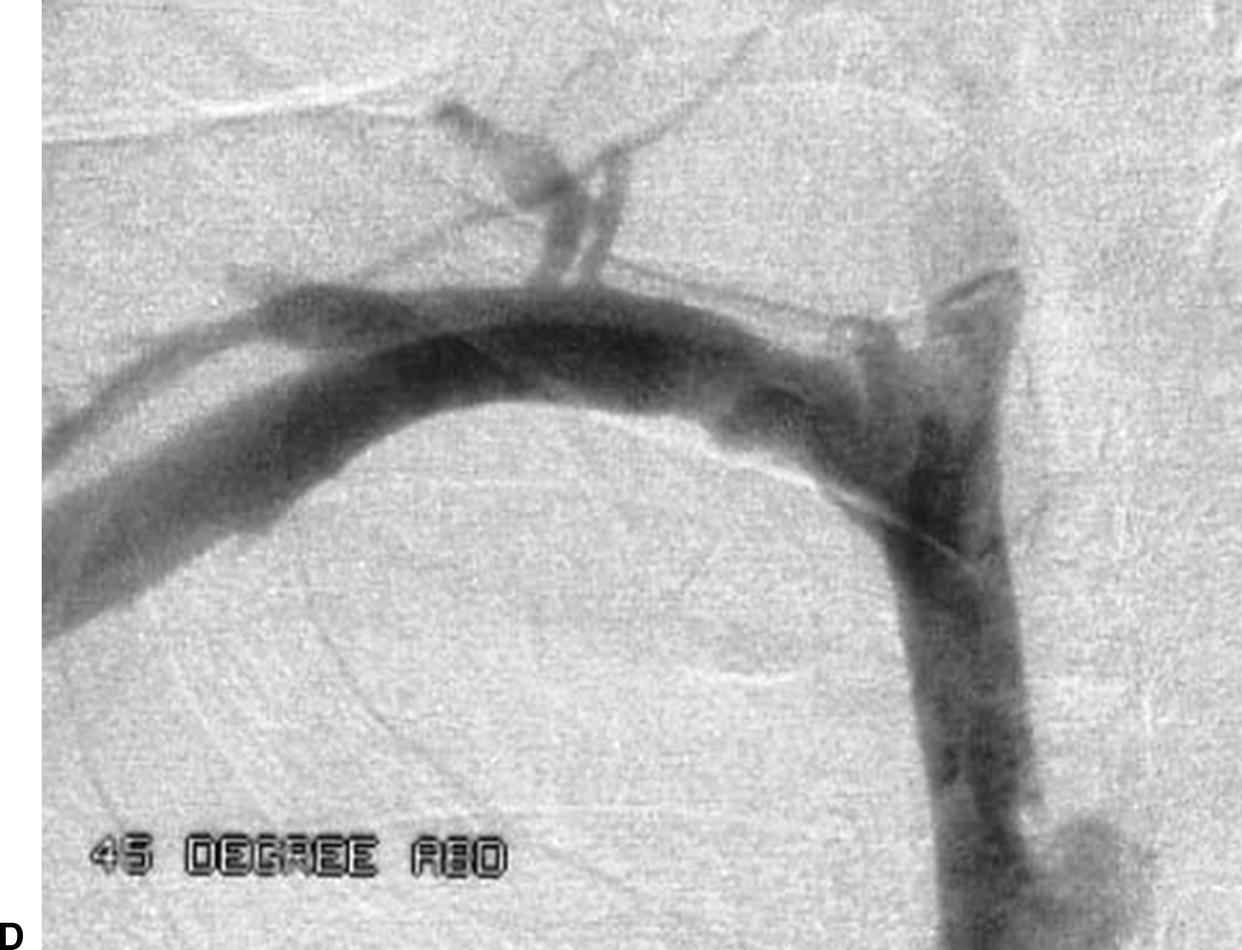
FIGURE 20.2. Case of effort thrombosis. A,Initial duplex evaluation shows acute thrombosis of the right subclavian vein with no color Doppler flow. B,Initial venogram shows near occlusion of the right subclavian vein (arrow). C,Postthrombolysis B-mode image of the right subclavian vein shows partial residual webbing and a “frozen” valve cusp in the lumen. D,Final venogram shows a patent right subclavian vein postthrombolysis.
Following thrombolysis, the patient will generally fall into one of these four categories based on the appearance of the postlysis venogram.
1. Complete recanalization with no evidence of residual narrowing with the arm in neutral position or in one of the provocative positions. This is relatively unusual. These patients should be anticoagulated and worked up for hypercoagulable states and occult malignancy. Thoracic outlet decompression is not indicated.
2. Complete recanalization with evidence for extrinsic compression, particularly with provocative arm/shoulder positioning. The treatment of these patients is somewhat controversial. Some advocate anticoagulation alone for these patients while many surgeons perform immediate or delayed thoracic outlet decompression, usually requiring some combination of first rib removal, scalenotomy, and resection of the costoclavicular ligament done via a supraclavicular, infraclavicular, or transaxillary approach. In addition, some of these patients have cervical ribs that require resection.
3. Complete or near-complete recanalization with evidence for both extrinsic and intrinsic venous compromise. The intrinsic compromise is due to residual thrombus, scar tissue, or both. If the intrinsic compromise is severe and does not improve substantially with balloon angioplasty, many advocate thoracic outlet decompression coupled with venous repair involving combinations of endovenectomy, vein patch angioplasty, interposition vein grafting, or jugular vein “turn down.” Venous repair usually requires a combined supraclavicular and infraclavicular approach or partial claviculectomy. The use of stents to treat residual stenoses in the axillosubclavian venous segment is generally contraindicated due to high rates of stent fracture, recurrent stenosis, and thrombosis.40
4. Failed thrombolysis with continued axillosubclavian vein occlusion. These patients should be anticoagulated. Many will experience complete or near-complete resolution of symptoms. For those with continued severe symptoms and reconstructable venous anatomy, a venous reconstruction with thoracic outlet decompression should be considered.
5. There are many similarities between upper and lower extremity DVT, as well as some important differences. Many aspects of the diagnosis and treatment of upper extremity DVT require further study including: the role of clinical probability scoring systems, the accuracy of duplex scanning using state-of-the-art equipment and scanning techniques, the role of novel anticoagulants such as the direct thrombin inhibitors and factor Xa antagonists, the optimal duration of anticoagulation as determined by risk stratification on the basis of competing risks of rethrombosis off anticoagulation versus major bleeding risk on anticoagulation, the role of anticoagulation for patients with upper extremity superficial venous thrombophlebitis and brachial and forearm DVT, the role of prophylactic therapy in selected high-risk populations for preventing upper extremity DVT, and optimal criteria for selecting patients for thrombolysis and thoracic outlet decompression.
DUPLEX SCANNING
Instrumentation
A standard duplex ultrasound system is used for the upper extremity venous examination with high-resolution B-mode imaging, color Doppler, and spectral waveform analysis. A midrange-frequency curved transducer (5 to 8 MHz) with a small footprint (as used in neonatal head examinations) is helpful when evaluating the subclavian vein, because access is limited by the clavicle. A high-frequency transducer (10 to 15 MHz) may be helpful for evaluating very superficial veins. Some of the common transducer configurations and their sector image patterns are shown in Figure 20.3.
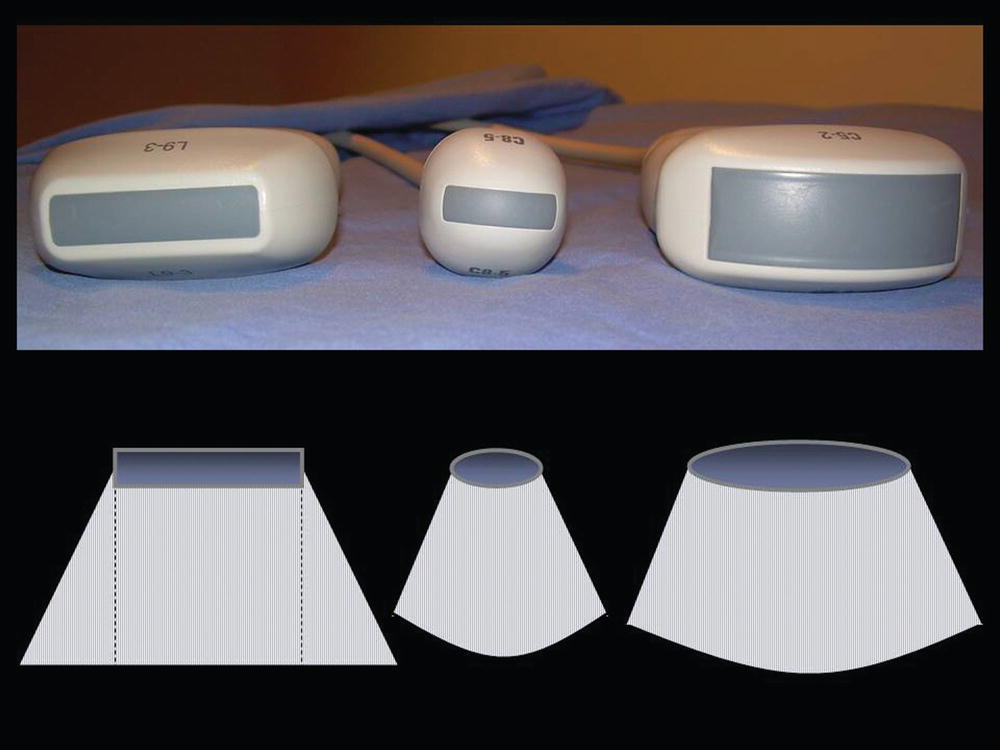
FIGURE 20.3. Examples of various transducer shapes and sector image patterns for scanning of the central and peripheral upper extremity veins.
Venous duplex imaging requires the parameters for color Doppler and Doppler spectral waveforms to be set for optimal detection of low velocity flow. The wall filter should be set at the lowest setting to avoid cutting off low flow velocities. Increasing power and gain maximizes the Doppler flow signal with increasing depth through the tissue. However, overcompensation of the Doppler gain settings produces a mirror image of flow signals in the spectral display above and below the zero baseline that will be corrected by decreasing the gain. Spectral Doppler and color Doppler settings should be set to optimize peak velocities in the range of 50 to 100 cm/s. The upper extremity venous duplex protocol is summarized in Table 20.3.
TABLE 20.3 Upper Extremity Venous Duplex Protocol
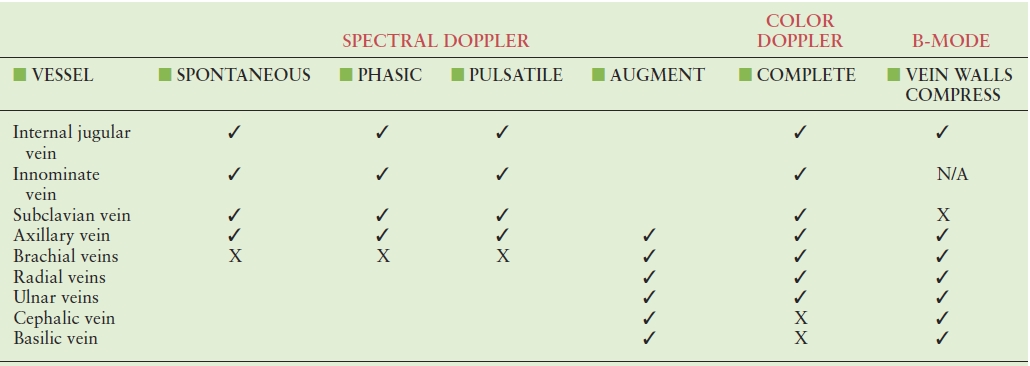
✓, routine; X, when applicable; N/A, not applicable.
Patient Position
For scanning of the central upper extremity veins, it is best for the patient to be supine with the neck turned to the contralateral side and the chin slightly raised, as shown in Figure 20.4. However, if the neck is turned too far to the contralateral side, the sternocleidomastoid muscle will tighten across the neck, making transducer contact difficult and vein wall compression nearly impossible. This is also an uncomfortable position for the patient. If the patient is sitting up, the veins may collapse, making imaging more difficult. Conversely, if the patient’s head is positioned lower than the heart, the veins (particularly the internal jugular vein) may become dilated and difficult to compress.
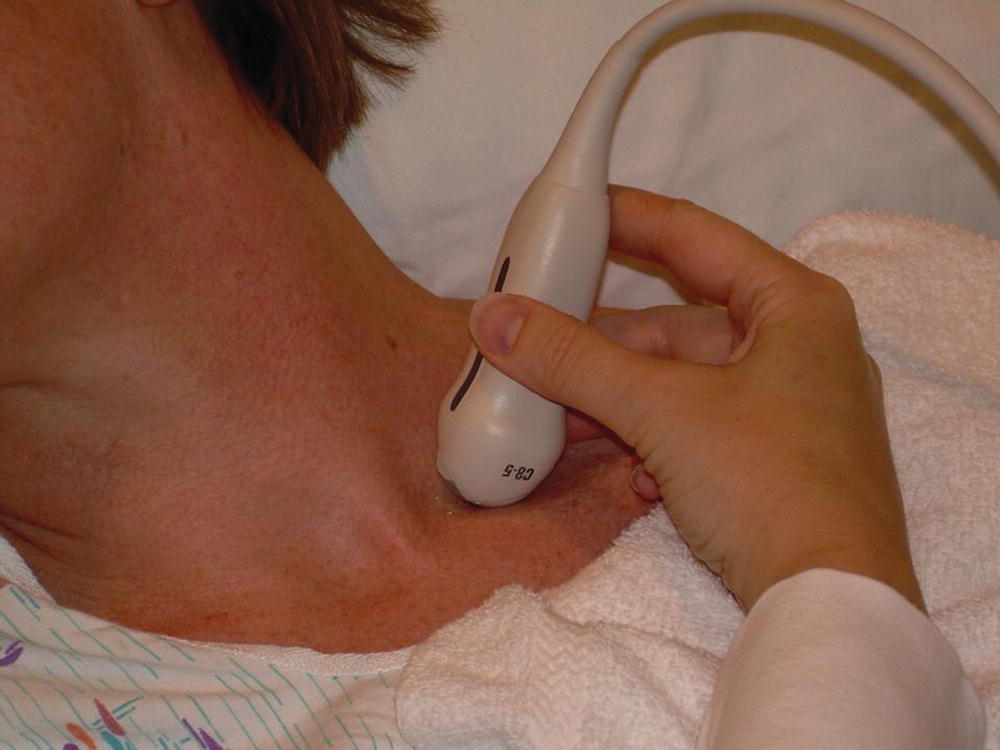
FIGURE 20.4. Patient position for left innominate and proximal subclavian vein evaluation. The transducer is positioned in the supraclavicular fossa.
Deep Venous Anatomy
The internal jugular, innominate or brachiocephalic, and subclavian veins are the central veins that drain the arms and head, as shown in Figure 20.5.
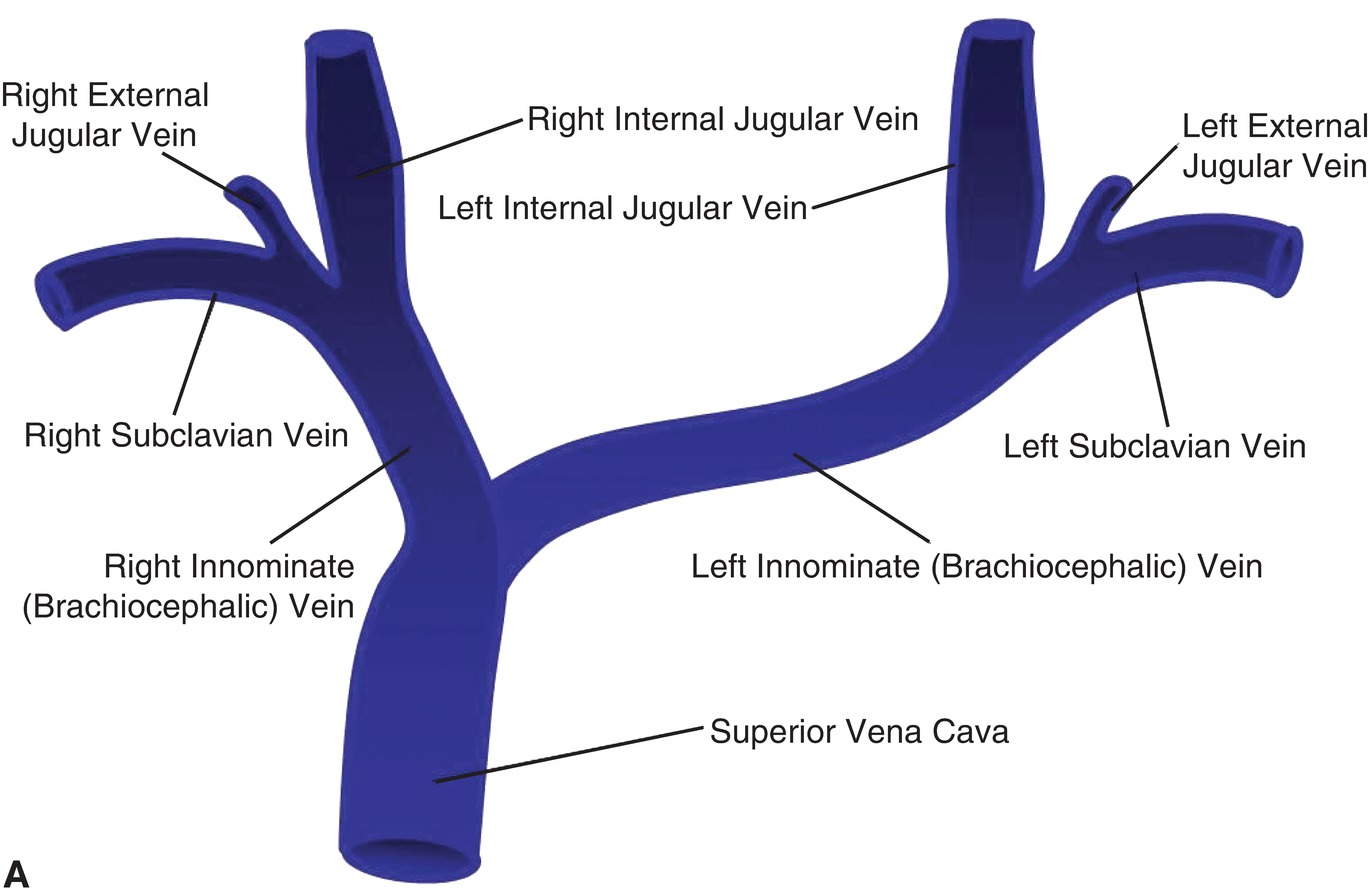
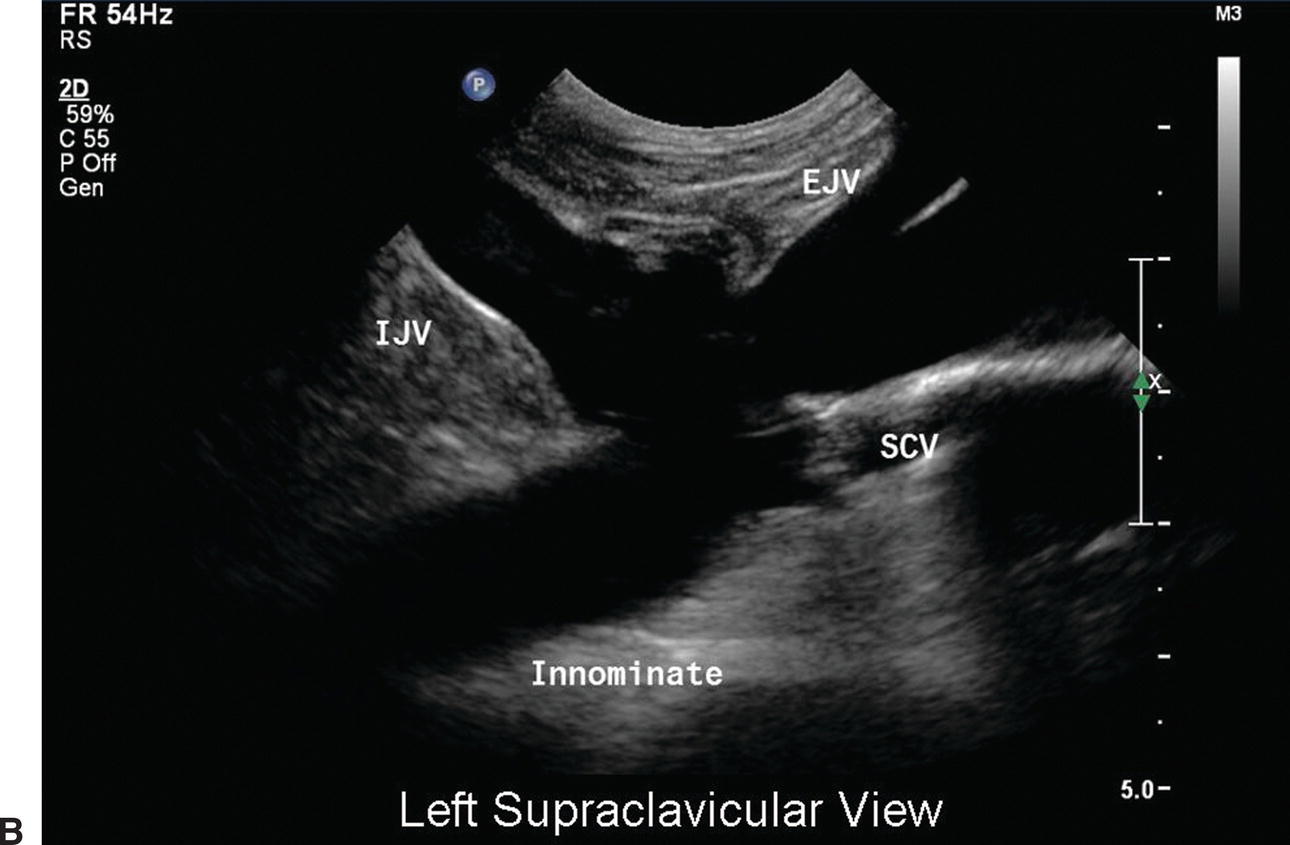
FIGURE 20.5. A,The upper extremity central veins. B,Corresponding B-mode image of the left upper extremity central veins. EJV, external jugular vein; IJV, internal jugular vein; SCV, subclavian vein.
Internal Jugular Veins
The internal jugular vein drains blood from the brain, the face, and the neck. It begins at the jugular foramen at the base of the skull where it is often somewhat dilated, and this dilatation is called the “superior bulb.” It also has a common trunk that receives drainage from the anterior branch of the retromandibular vein, the facial vein, and the lingual vein. The internal jugular vein courses vertically down the neck parallel to the internal carotid and common carotid arteries. At the base of the neck, it joins the subclavian vein to form the brachiocephalic (innominate) vein, and just above this confluence is a second dilatation, termed the “inferior bulb.” At the base of the neck, the right internal jugular vein is often more lateral than the common carotid artery, and it crosses over the proximal subclavian artery, whereas the left internal jugular vein usually overlaps the common carotid artery. The left internal jugular vein is typically smaller than the right, and each contains a valve about 2.5 cm above the central end of the vessel.
The internal jugular veins are relatively superficial and not protected by bone or cartilage, making them susceptible to injury. There are no valves between the right atrium and the internal jugular vein, so blood can flow back into the internal jugular when the pressure in the right atrium is high. This can be seen externally as a pulsation at the base of the neck with the patient in the supine position, the head of the bed elevated at 45 degrees, and the head turned slightly to the contralateral side. However, unlike the carotid pulse, this jugular venous pulse is not readily palpated. Reasons for jugular venous pressure to be increased include right heart failure, tricuspid stenosis, tricuspid regurgitation, and cardiac tamponade.
Because the internal jugular vein is large, central, and relatively superficial, it is a common site for central line placement, and because it rarely varies in location, it is generally easier to cannulate than other veins. However, there is a risk of missing the vein and puncturing the adjacent carotid artery, especially when venous cannulation is performed without ultrasound guidance, as discussed in Chapter 27. A duplex evaluation of the neck may be requested following an attempted internal jugular vein puncture to evaluate for vascular injury if the patient develops severe swelling or a hematoma. Rarely, a pseudoaneurysm may be found arising from the common carotid or some other artery in the neck.
Brachiocephalic (Innominate) Veins
As previously mentioned, the brachiocephalic veins are also known as the innominate veins. The left and right brachiocephalic veins are formed by the union of the corresponding internal jugular and subclavian veins (see Fig. 20.5A). The term “brachiocephalic” originates from the combination of a Latin term brachium meaning “arm” and the Greek kephale meaning “head.” The right and left brachiocephalic veins join to form the SVC. Unlike the arterial system in which there is typically one innominate artery (on the right), the upper extremity venous system comprises two innominate veins (right and left). The innominate veins are best visualized using a low-frequency transducer scanning in the supraclavicular space with the vein coursing directly away from the transducer (see Figs. 20.4 and 20.5B). The left innominate vein may be more difficult to image because it takes a longer course than the right innominate vein, crossing the midline toward the right side of the heart.
The right brachiocephalic vein passes almost vertically downward in front of the innominate artery, and the left brachiocephalic vein passes from left to right behind the upper part of the sternum. Each of these veins receives a vertebral, deep cervical, deep thyroid, and internal thoracic vein. The left brachiocephalic vein also receives intercostal, thymic, tracheal, esophageal, phrenic, mediastinal, and pericardiac branches.
Subclavian Veins
The subclavian vein is a continuation of the axillary vein and runs from the outer border of the first rib to the medial border of the anterior scalene muscle (Fig. 20.6). The subclavian vein lies anterior and inferior to the subclavian artery as it crosses the first rib; they are separated by the anterior scalene insertion. Anteriorly, the subclavian vein is covered throughout its entire course by the clavicle. Initially, the vein arches upward across the first rib before turning medially, downward, and slightly forward across the insertion of the anterior scalene muscle to enter the thorax where it joins with the internal jugular vein behind the sternoclavicular joint. The thoracic duct drains into the left subclavian vein near its junction with the left internal jugular vein; the right subclavian vein receives the right lymphatic duct.
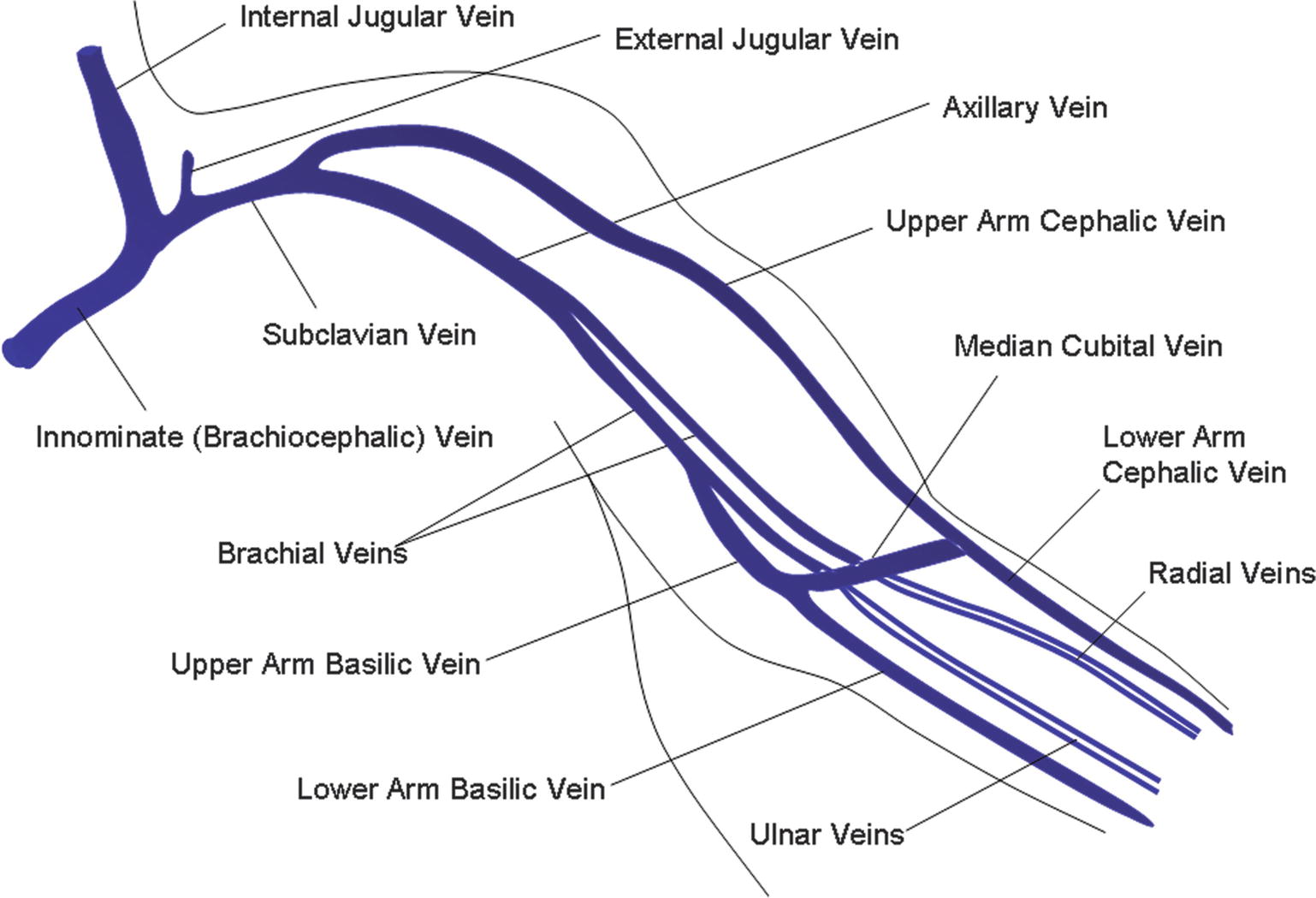
FIGURE 20.6. The upper extremity veins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


