FIGURE 1.1. When patients develop microemboli that pass down to the plantar and digital arteries, the appearance of the foot may be a useful clue to the underlying process. This patient had embolized cholesterol crystals from ulcerated plaques to both feet. He presented with pain, coolness, and this very prominent livedo reticularis pattern of skin mottling. Transmetatarsal amputations were required to control the pain. The cholesterol crystals were seen by polarized light and were the cause of the arterial occlusion and ischemia.
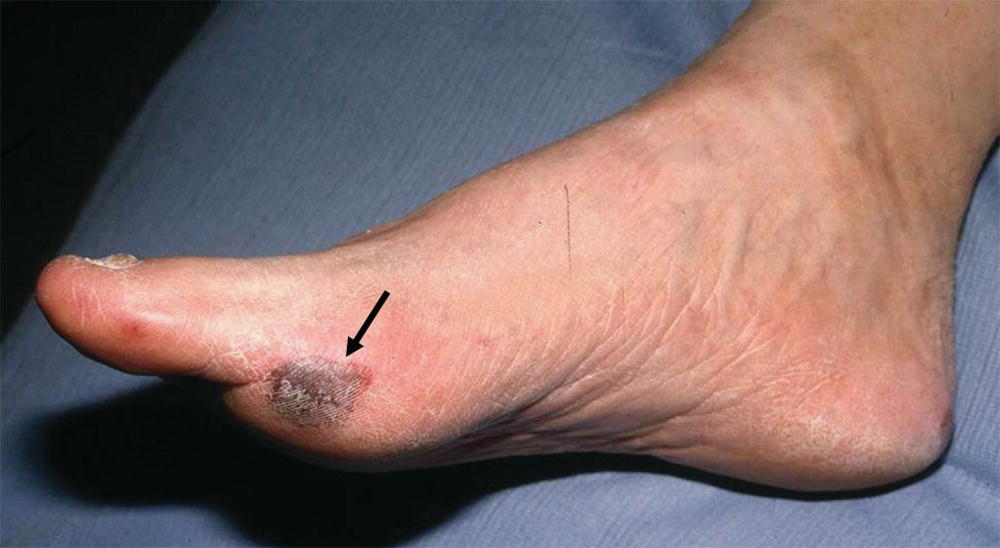
FIGURE 1.2. A focal area of skin infarction (black arrow) on the first metatarsal head caused by a small atheroembolus.
A detailed history with regard to symptoms before the acute event is important. If the patient gives a history of intermittent claudication, this is evidence that the patient has chronic arterial occlusive disease, with the current acute episode representing a thrombosis in the region of a preexisting high-grade stenosis. In the course of the physical examination, look for the presence of an aortic, femoral, or popliteal artery aneurysm that could be the source of emboli or thrombosis as a cause of acute limb ischemia.
In patients who have palpable pulses to the level of the foot and appear to have microemboli as the cause of ischemia, listen for bruits from the level of the abdominal aorta to the popliteal fossa. Very few providers regularly listen over the superficial femoral and popliteal arteries for bruits, and Dr. Strandness would insist that this part of the physical examination be included in patients with suspected microemboli. The presence of a bruit indicates turbulent blood flow and a possible site of stenosis. Dr. Strandness told his residents that bruits are transmitted downstream from their site of origin, not upstream; “they go with the flow” was what he would say. Use a continuous wave (CW) Doppler to determine whether audible arterial flow is present in the tibial arteries. Dr. Strandness also considered ankle pressure measurement and calculation of the ankle-brachial index to be part of the routine vascular physical examination. He would often take the time to perform this simple test himself, even when a vascular technologist was available (Fig. 1.3).
FIGURE 1.3. Dr. Strandness measuring an ankle pressure in the 1960s.
The causes of acute ischemia in the upper extremity are similar to those found in the lower extremity, but some differences can be noted. Atherosclerosis of the arteries supplying the arm tends to involve only the innominate artery and the first part of the subclavian artery. Thus, acute ischemia secondary to thrombosis of an atherosclerotic plaque is very unusual. The principal cause of acute upper extremity ischemia is emboli, which most often arise from the heart. An infrequent source of emboli is a lesion in the subclavian artery at the thoracic outlet. Chronic compression of the subclavian artery at that site can lead to intimal damage with subsequent thrombosis and release of emboli. These events typically occur in younger patients who are in good health. Therefore, in any patient who suddenly develops acute ischemia of the hand, suspect damage to the subclavian artery from repeated compression.
Other causes of acute hand ischemia are to be found among those disorders that include vasospasm, such as Raynaud’s syndrome, Buerger’s disease, scleroderma, and mixed connective tissue disorders, as discussed later in this chapter. Although vasospasm is usually a chronic problem, there are cases in which thrombosis of the digital and palmar arteries can lead to acute ischemia and gangrene. Dr. Strandness was known for his expertise in dealing with primary and secondary vasospastic disorders, and the most common underlying condition in his experience that was associated with this problem was scleroderma (Fig. 1.4). Many of the principles relating to the evaluation of acute ischemia of the hands are similar to those employed for the lower limb (see Chapters 12, 13, and 14).
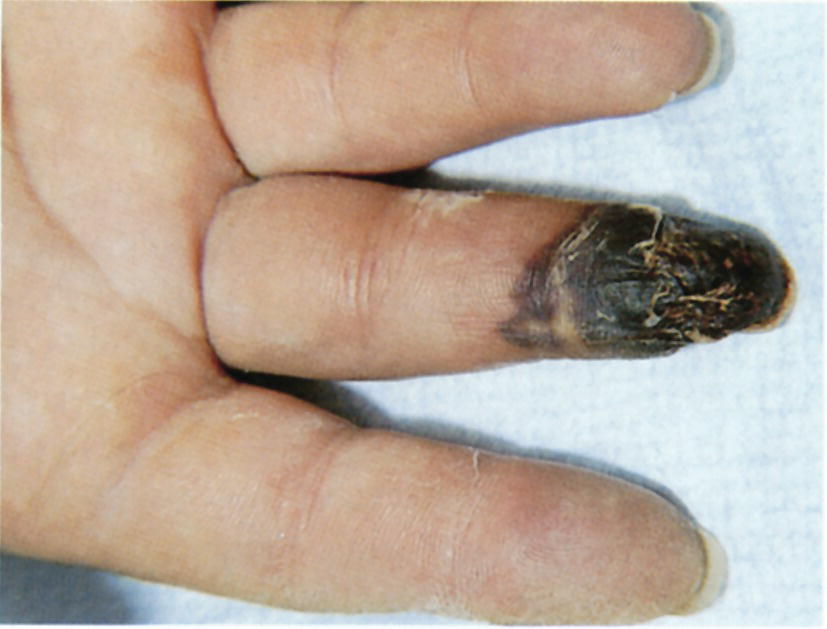
FIGURE 1.4. Patients with scleroderma may develop gangrene of the fingers. This is a result of thrombosis and occlusion of the digital arteries.
Chronic Arterial Occlusion
The classic symptom of chronic peripheral arterial disease is intermittent claudication. This crampy muscular pain may be described by the patient as aching, fatigue, weakness, or even numbness in the extremity. However, the key feature of claudication is that it is consistently produced by the same intensity of leg exercise or activity and relieved with a few minutes of rest. It may gradually improve or get worse over months and years, but it is relatively constant from day to day. The symptoms are caused by the inability of the diseased arteries and collateral vessels to provide adequate blood flow to the tissues in the face of increased demands for nutrients and oxygen during exercise. The sites of the responsible arterial lesions can be deduced from the location of claudication because the symptoms occur in muscle groups distal to the occlusive disease. As the arterial lesions progress, patients may notice that their comfortable walking distance is shorter, or they may experience increased pain with ambulation. McLafferty and coworkers8 found that 50% to 70% of patients over 70 years of age with claudication perceived their discomfort to be a normal part of aging and did not report the symptoms to their providers.
Patients with unusual or atypical leg pain are commonly referred to a vascular specialist and the vascular laboratory for evaluation. These patients are often considered to have “pseudoclaudication” if no evidence of significant arterial occlusive disease is found. The most common nonvascular conditions responsible for such a presentation are neurospinal and orthopedic problems. The patient with pseudoclaudication frequently describes a walk-pain cycle that is not constant from day to day, for example, being able to walk a mile one day but limited to a few blocks the next day. In addition, the pain may be brought on by standing or sitting for prolonged periods of time, or the patient may have to lie down to obtain complete relief.
When arterial insufficiency becomes severe, the patient may have persistent pain in the distal foot and toes at rest. This “rest pain” indicates a critical state of ischemia, because it signifies that the tissue blood supply is inadequate at all times, not just during limb exercise. Rest pain is often worse at night and may interfere with sleep. Placing the limb in a dependent position typically reduces rest pain by producing a modest increase in distal limb blood flow. Changes resulting from chronic severe ischemia that can be noted on physical examination include loss of hair, brittle nails, dry or scaling skin, muscle atrophy, and ulcerations. Lower extremity edema may be apparent bilaterally or unilaterally and is usually caused by prolonged periods of dependency related to ischemic rest pain. Gangrenous changes or tissue necrosis appear after prolonged severe ischemia. In elderly patients who are inactive, gangrene may be the first sign of arterial occlusive disease (Fig. 1.5). These patients may have adjusted their lifestyle to accommodate the limitations imposed by their disease and may never walk far enough to develop symptoms of claudication. Although their circulation is severely impaired, this is not apparent until ulceration develops.
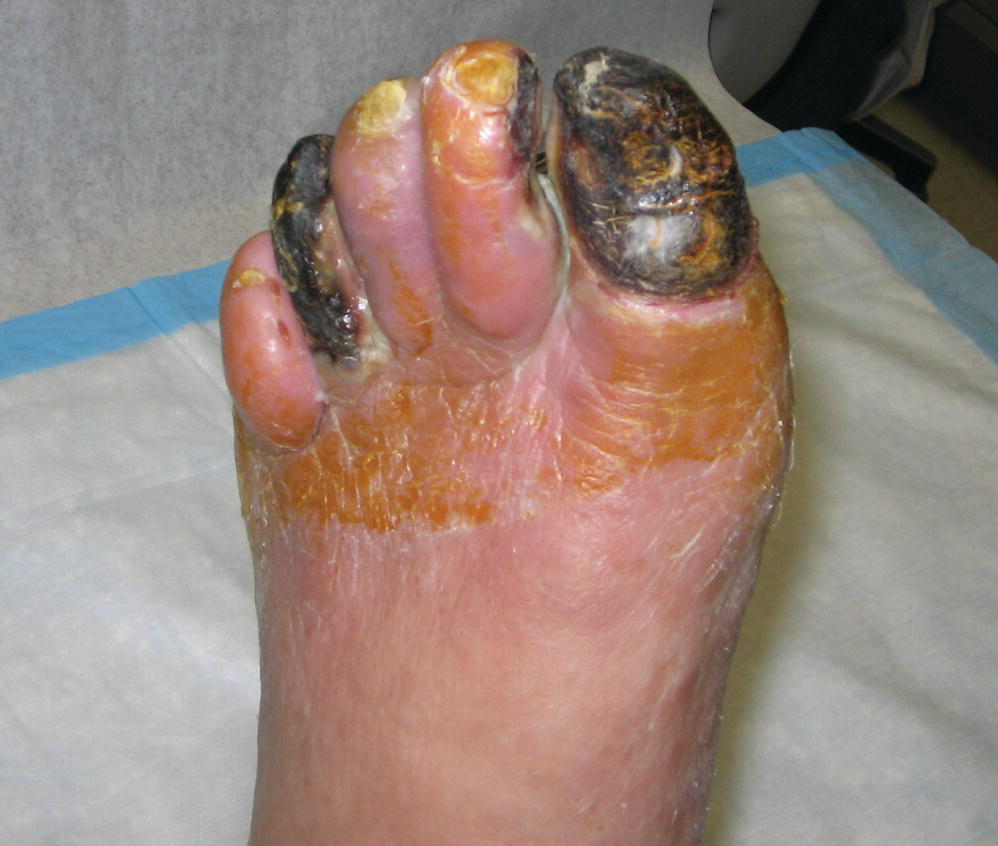
FIGURE 1.5. Necrosis or gangrene of several toes due to severe arterial occlusive disease in an elderly nonambulatory patient.
Arterial occlusive disease can reduce or obliterate pulsations in the extremities. The level of pulse deficit is a good marker for the most proximal level of involvement. Palpate pulses bilaterally and simultaneously, if possible, comparing both sides for symmetry in rate, rhythm, and quality. Palpation of pulses is subjective, and the examiner may mistake their own pulse for that of the patient. To prevent this, the examiner should apply light pressure and avoid using only the index finger for palpation because this finger has the strongest arterial pulsation of all the fingers. The thumb also has a strong intrinsic pulsation and should not be used for pulse palpation.
Dr. Strandness was insistent that all vascular patients should have a thorough evaluation of their feet for trophic changes, especially for evidence of emboli or ulcerations. One of Dr. Strandness’ favorite stories was about a medical student who had been in to see a patient who had been followed for years in the clinic. The medical student gave his presentation and stated that the patient had normal lower extremity pulses bilaterally. Dr. Strandness did not say a word but went into the examination room with the medical student and pulled up the gentleman’s trousers, noting that the patient still had his socks and shoes on, and asked the student how he was able to palpate pulses through the shoes. The next thing he did was ask the patient to remove his below-knee prosthetic limb so he could assess the patient’s stump site. Dr. Strandness did not tell this story to make fun of the student (which the student clearly deserved), but rather to emphasize that all patients must have their socks and shoes removed for the physical examination.
Chronic arterial occlusive disease occurs less frequently in the upper extremities than in the lower extremities and causes less severe symptoms because the collateral circulation tends to be significantly better in the arms. The arms also have less muscle mass and are not subjected to the same heavy workload as the legs. As previously mentioned, upper extremity atherosclerotic lesions typically occur proximal to the origin of the vertebral artery, setting up the vertebral artery as a major contributor to collateral flow. When upper extremity arterial occlusive disease is symptomatic, the patient complains of arm fatigue and pain with exercise (arm claudication) and inability to hold or grasp objects (e.g., painting, combing hair, using hand tools, placing objects on shelves above the head, and occasionally difficulty driving). Dr. Strandness would stress to the vascular technologists that they should ask the patient about the specific activities that produced his or her symptoms and try to perform an evaluation under similar conditions. Many patients with symptomatic upper extremity arterial occlusive disease do not present with classic claudication. This condition may cause vertebrobasilar symptoms such as vertigo, ataxia, syncope, or bilateral visual changes. Significant findings on physical examination include coolness and pallor of the affected extremity, decreased capillary refill of the digits, and a difference in brachial systolic blood pressures of more than 10 to 15 mm Hg.
One of Dr. Strandness’ patients was a minister from a rural area who drove many miles daily visiting his parishioners. He initially presented to vascular surgery clinic for treatment of uncontrolled hypertension and renal artery stenosis. Once his renal artery disease had been treated and his blood pressure was under control, he began noticing that when driving and turning his head side to side he would become dizzy, and if he tilted his head, he developed severe vertigo. Duplex ultrasound evaluation showed that he had bilateral carotid artery disease and a subclavian artery occlusion. Physiologic noninvasive testing revealed that his upper extremity was stealing from his vertebrobasilar system when he drove long distances. What was most interesting about this patient was that when he was hypertensive, he appeared to have enough perfusion to his posterior circulation to be symptom free, but when his blood pressure was lowered to normal levels, he became symptomatic.
VASOSPASM
Patients who develop digital ischemia when their hands or feet are exposed to cold are often sent to the vascular laboratory for evaluation. This presentation is referred to as Raynaud’s syndrome and can be divided into primary Raynaud’s phenomenon and secondary Raynaud’s disease. Raynaud’s phenomenon is a common clinical disorder characterized by intermittent episodes of vasoconstriction of the small arteries of the hands and feet that cause dramatic color and temperature changes. Patients with Raynaud’s phenomenon have a young age of onset (usually <30 years), and the episodes are associated with minimal pain and typically involve all digits in a bilateral and symmetrical fashion. In this primary benign form, the palmar and digital arteries remain patent without any demonstrable structural abnormalities. Epidemiologic studies indicate that the prevalence of Raynaud’s phenomenon is between 6% and 20% in women and 3% and 13% in men. There is also a possible genetic susceptibility to the condition.9
By contrast, patients with Raynaud’s disease have asymmetrical digit involvement and intense pain related to an underlying pathologic condition (Fig. 1.6). This secondary form is more serious because it is associated with intrinsic occlusive lesions in the digital arteries and a long list of systemic diseases, of which the collagen-vascular diseases are the most common. Secondary Raynaud’s disease can also occur in the setting of arterial occlusions proximal to the wrist, hematologic syndromes, infectious diseases, and in patients with repetitive trauma from vibrating tools or anatomic abnormalities such as a cervical rib. Raynaud’s disease is a form of intermittent arteriolar vasoconstriction that results in coldness, pain, and pallor of the fingertips or toes. Symptoms may result from a defect in basal heat production that eventually decreases the ability of cutaneous vessels to dilate. Episodes may be triggered by emotional factors or unusual sensitivity to cold.
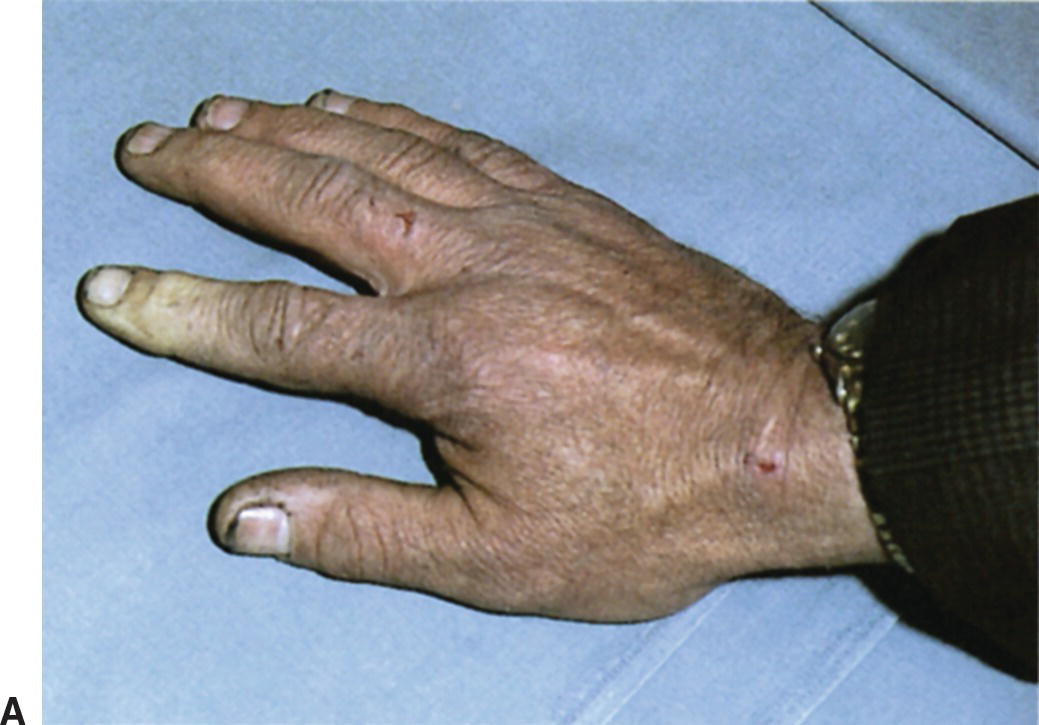
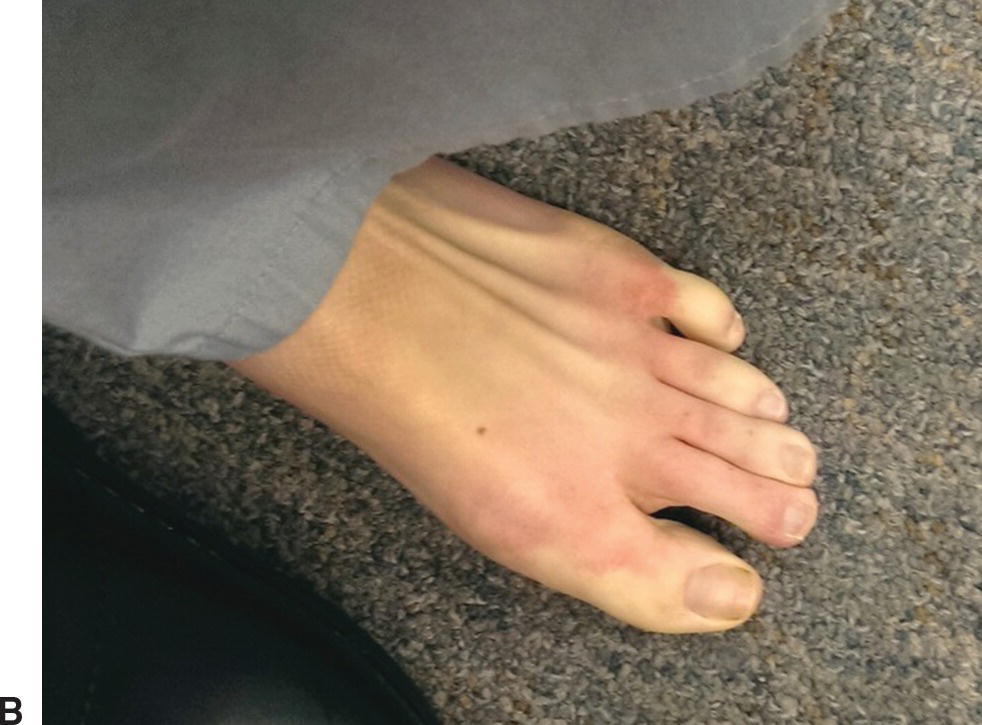
FIGURE 1.6. (A) This patient had secondary Raynaud’s disease with digital artery occlusion and ischemia confined to the index finger of the right hand. (B) Another patient with Raynaud’s disease and pallor involving the toes.
The classic clinical picture consists of three phases and begins with pallor of the fingers or toes brought on by sudden intense vasoconstriction. The skin then becomes bluish (cyanotic) due to pooling of deoxygenated blood in the tissues. The final phase occurs as vasospasm resolves and the small arteries vasodilate, producing a hyperemic state. This results in a reddish color (rubor) as oxygenated blood returns to the digits. The characteristic sequence of color changes in cold sensitivity of the Raynaud type is described as “pallor, cyanosis, and rubor” or “white, blue, and red.” Numbness, tingling, and burning pain may occur as the colors change. The primary role of the vascular laboratory is to determine the anatomic status of the circulation to the digits and assess their response to cold exposure (see Chapter 14). The physical examination is usually not helpful unless digit ulceration is noted.
Buerger’s disease is one of the systemic disorders associated with the development of cold sensitivity and vasospasm. It is regarded as an autoimmune vasculitis and occurs most often in men between 20 and 35 years of age. Considerable evidence indicates that heavy smoking or chewing of tobacco is a causative or aggravating factor.10 The vasospastic manifestations of Buerger’s disease are generally bilateral and symmetrical, with focal occlusive lesions in the small arteries. Pain is the outstanding symptom, and it is often aggravated by nicotine, cold exposure, or emotional disturbances.
ABDOMINAL AORTIC ANEURYSM
An aneurysm is a permanent localized dilatation of an artery. Symptoms are variable and depend on how rapidly the artery dilates and how the pulsating mass affects surrounding structures. Many patients are asymptomatic, and the aneurysm is found incidentally during a workup for some other problem. A history of smoking is the risk factor most strongly associated with an abdominal aortic aneurysm (AAA), but other factors such as having a first-degree relative with an AAA and having atherosclerosis are also independent risk factors.11 Most aneurysms in the abdominal aorta occur below the level of the renal arteries. AAAs are more common among Caucasians, affecting men four to six times more often than women.12 Some patients complain that they can feel their heart beating in their abdomen when lying down, or they may describe a “throbbing” feeling in their abdomen.
The most important diagnostic sign of an AAA is a palpable pulsatile mass in the epigastric area or upper abdomen. A systolic bruit may be auscultated over the mass. The physical examination should include palpation of the abdominal aorta and the iliac, femoral, and popliteal arteries to determine whether there is a bounding pulse. As has been previously mentioned, if an AAA is present or suspected, evaluate the toes for evidence of embolization. When a small (3 to 4 cm in diameter) AAA is identified, serial ultrasound follow-up is often recommended at 6- to 12-month intervals until the aneurysm reaches a size at which intervention is justified—typically 5 to 6 cm in diameter. An aneurysm that shows rapid growth or becomes symptomatic warrants an urgent vascular consultation.
Aneurysms may also arise in the peripheral arteries. The most frequent site for peripheral aneurysms is the popliteal artery, but other arterial sites such as the subclavian, renal, or common femoral arteries can also be involved. Between 50% and 60% of popliteal aneurysms are bilateral and may be associated with AAAs. If a bounding popliteal pulse is palpated, perform a duplex ultrasound to rule out a popliteal artery aneurysm. Aortic and peripheral artery aneurysms are discussed further in Chapter 15, and point-of-care screening for AAA is covered in Chapter 27.
AORTIC DISSECTION
When an atherosclerotic aorta develops a tear in the intima or the media degenerates, a dissection may result. Arterial dissections are three times more frequent in men than in women and occur most commonly in the 50- to 70-year-old age group.13 They are associated with poorly controlled hypertension, blunt chest trauma, and cocaine or methamphetamine use. A rupture may occur through the dissection flap back into the true lumen, allowing blood to reenter the main channel and resulting in a chronic dissection or causing occlusion of the branch arteries supplying the gastrointestinal tract, kidneys, spinal cord, or legs.
When aortic dissection occurs, onset of symptoms is usually sudden, with severe and persistent pain, often described as tearing or ripping. The location of the pain can include the anterior chest and shoulder region, interscapular area in the back, epigastric region, or abdomen, depending on the location and extent of the dissection. The patient may appear pale and sweaty, and tachycardia may be present. Blood pressure may be markedly different from one arm to the other if the dissection involves the orifice of the subclavian artery on one side. Because of the variable clinical picture associated with this condition, early diagnosis is usually difficult, and it may be mistaken for an acute myocardial infarction.
EXTRACRANIAL CEREBROVASCULAR DISEASE
In the United States, more than 790,000 strokes occur each year, of which about 185,000 are recurrent and 130,000 are fatal. One-quarter of the initial stroke survivors suffer a second stroke within 5 years.14 Stroke remains the third most common cause of death in the Western world, with atheroembolism from the carotid artery accounting for as many as half of all these events. According to the Atherosclerosis Risk in Communities Study from the American Heart Association, approximately 83% of strokes are ischemic and 17% are hemorrhagic.15 Most strokes occur in individuals older than 65 years, and women have 60,000 more strokes each year than men.14 In the prospective Ischemic Stroke Genetics Study, there were no sex differences in stroke severity, stroke subtype, or infarct size and location in patients with ischemic strokes.16 It has been shown that ethnic and racial minorities have an increased risk of stroke, and African Americans have almost twice as many strokes as Caucasians. African Americans of both sexes and women die at much higher rates than white men.12
Many risk factors for cerebrovascular disease are similar to those for atherosclerotic disease in other vascular territories; however, known coronary artery disease doubles stroke risk, and patients with atrial fibrillation have a risk five times greater. Hypertension (blood pressure >120/80 mm Hg) can double the lifetime risk of stroke.14 Diabetes mellitus, or even prediabetes (impaired glucose tolerance), is an independent risk factor for an ischemic stroke or transient ischemic attack (TIA).16 Additional risk factors associated with stroke are tobacco use, heavy alcohol consumption, hyperlipidemia, migraines, and pregnancy.14,16
Carotid atherosclerosis may reduce blood flow to the brain either by obstructing the lumen of the extracranial internal carotid artery or by embolizing to the intracranial arteries. The subtypes of arterial disease related to ischemic stroke are large-vessel disease, lacunar or small-vessel disease, and embolic stroke. In large-vessel disease, an atherosclerotic plaque can rupture, develop a thrombus, and give rise to an embolus that obstructs cerebral flow. Other large-vessel strokes may be caused by a severe stenosis or occlusion of the internal carotid artery, leading to ischemic injury downstream, also known as watershed infarction. Lacunar or small-vessel disease strokes usually involve small areas of infarction and have been associated with arteritis. Embolic strokes result from thrombi that form in the heart or elsewhere in the body, such as fat emboli related to long bone fractures or particulate emboli from intravenous drug abuse. In younger patients, strokes have also been attributed to arterial dissection, hypercoagulable states, sickle cell disease, and a patent foramen ovale (paradoxical embolism).14
Hemorrhagic strokes are associated with a higher mortality rate than ischemic strokes and are divided into two subtypes: intracranial hemorrhage and subarachnoid hemorrhage. Intracranial hemorrhage, defined as the presence of blood within the main cellular mass of the brain, accounts for 10% of all strokes and is associated with oral anticoagulant use and chronic hypertension. Subarachnoid hemorrhage accounts for 3% of all strokes and is more prevalent among middle-aged women, usually resulting from rupture of a cerebral aneurysm at a vessel bifurcation.14 Headache, decreased level of consciousness, and vomiting are extremely common with hemorrhagic strokes. The Rotterdam study of over 4000 subjects followed for an average of 10 years found that a decreased glomerular filtration rate (GFR) was an independent risk factor for hemorrhagic but not for ischemic stroke.17
Strokes may be categorized by the vascular territory involved, with the common distributions being middle cerebral artery (MCA), anterior cerebral artery (ACA), posterior cerebral artery (PCA), brainstem, cerebellum, and strokes occurring in more than one vascular territory. Many providers simplify this scheme and refer to either anterior or posterior circulation strokes. Typical symptoms of an anterior circulation stroke include weakness, numbness, or tingling on one side of the body, expressive or receptive aphasia, transient monocular blindness (amaurosis fugax), and difficulty reading or writing. These symptoms generally originate in the MCA and ACA distributions. If a patient experiences double or blurred vision, vertigo that may or may not be positional, and syncopal episodes, the stroke is more likely to be related to the vertebrobasilar system or posterior circulation (PCA, brainstem, cerebellum). Light-headedness and dizziness are very nonspecific and, in the absence of other symptoms, cannot be attributed to carotid artery disease. One prospective study of over 2000 patients found that the most common stroke categories were MCA (50.8%) and small-vessel (12.8%) stroke.18 This same study also found that patients with MCA and PCA strokes were older and those with strokes in multiple vascular beds were younger. The authors found no significant difference in the proportions of male to female patients with strokes in the various vascular territories.
Referrals to the vascular laboratory for a cerebrovascular duplex study include patients with neurologic symptoms, but many asymptomatic patients are referred after a bruit is found on physical examination. A bruit is only a sign that disease is present and cannot be used to determine the severity of the underlying arterial narrowing. Dr. Strandness was always quick to point out that a significant carotid stenosis can exist in the absence of a bruit. The physical examination should include evaluation of any neurologic deficit and palpation of carotid pulses to determine whether there is asymmetry, which would indicate a stenosis or occlusion. Auscultation should be done low in the neck, over the mid neck, and at the angle of the jaw to determine whether a carotid bruit is present. Auscultate the supraclavicular and infraclavicular spaces for bruits caused by lesions in the proximal aorta or the aortic arch branches. In addition, Dr. Strandness would stress the importance of listening for cardiac murmurs, especially if the symptoms suggested a possible embolic event. It is also important to measure the systolic blood pressure in both arms at the time the patient is first seen to determine whether subclavian steal syndrome is a possible diagnosis. In this setting, a high-grade stenosis or occlusion of one subclavian artery may result in reversal of flow in the ipsilateral vertebral artery, leading to posterior circulation symptoms. However, it must be pointed out that reversal of flow in the vertebral artery in and of itself is usually a benign finding.
RENOVASCULAR DISEASE
The clinical spectrum of chronic kidney disease (CKD) ranges from a mild decrease in GFR to end-stage renal failure. Stages of CKD are based on levels of estimated GFR, and most renal risk from atherosclerosis occurs at stage III CKD (estimated GFR <60 mL/min/1.73 m2). It is estimated that renal artery stenosis is present in 2.1% of all new cases of end-stage renal failure.12 Occlusive disease of the renal arteries may result in hypertension or ischemic nephropathy. Ischemic nephropathy is defined as a progressive decline in renal function secondary to global renal ischemia. CKD has effects that have been shown to harm the cardiovascular system, including inhibition of erythropoiesis and platelet function, induction of volume overload, dyslipidemia, hypertension, and vascular calcification.17
In evaluating claims data from over 1 million Medicare patients from 1999 to 2001, Kalra and colleagues19 found that the prevalence of symptomatic atherosclerotic renovascular disease in the US Medicare population was approximately 0.5% overall and 5.5% in those with CKD. Since early 2000, atherosclerotic renovascular disease has been noted more commonly in older patients beginning dialysis therapy. The comorbid factors associated with a greater likelihood of atherosclerotic renovascular disease are peripheral arterial disease and atherosclerotic heart disease.20 Other risk factors identified include hypertension, African American race, age older than 85 years, diabetes, obesity, excretory renal insufficiency, and low high-density lipoprotein (HDL) cholesterol level.21 African American patients tend to develop end-stage renal failure a decade earlier than Caucasians and are less likely to have renal artery stenosis.12
As discussed in Chapter 24, the principal lesions associated with renal artery stenosis are atherosclerosis and fibromuscular dysplasia (FMD). Atherosclerotic lesions are usually ostial, involving the origin and proximal segments of the renal artery along with the adjacent aorta. Renal artery stenosis is present in approximately 5% to 10% of individuals aged 65 years or older, and the prevalence increases to 20% to 30% in high-risk subsets.22 Atherosclerotic renal artery stenosis is a progressive disease that reduces blood flow to the kidneys and activates the renin-angiotensin-aldosterone system. This system preserves renal blood flow by the production of angiotensin II, resulting in renal arteriolar vasoconstriction and stimulation of sodium reabsorption, leading to increased blood flow through the kidney and maintenance of GFR.23 Progression of renal artery disease leads to hypertension, renal dysfunction, and ultimately end-stage renal failure; it is most likely to occur in patients with preexisting high-grade renal artery stenosis, elevated systolic blood pressure, and diabetes mellitus.24
FMD is a nonatherosclerotic, noninflammatory arterial disease leading to segmental stenosis of the renal arteries. Three main types of FMD have been described: intimal, medial, and perimedial. The medial type is found in 85% of renal arteries with FMD.25 The lesions tend to be multifocal with a “string-of-beads” appearance forming a long irregular stenosis. Unlike atherosclerotic lesions, stenoses due to FMD rarely affect the ostial or proximal segments of the renal artery but are typically found in the mid and distal segments, and they may involve the renal artery branches. In most series, FMD accounts for only 10% to 20% of renal artery lesions, with atherosclerosis being the most common cause of renal artery stenosis.25 FMD is found mostly in women between 30 and 40 years of age, and it appears to have at least a partial genetic basis with an increased familial prevalence. Dr. Strandness had a special interest in renal artery FMD and followed three patients from the same family—two sisters and a brother—who all had this condition. The two sisters had renal, carotid, and mesenteric artery FMD, and the brother had renal and iliac artery involvement. He used these patients as teaching cases because the presence of FMD in more than one vascular territory is somewhat unusual, but he also considered them to be friends and loved to have them come into the clinic just to socialize.
The physical examination should include bilateral brachial stethoscope blood pressures to check for systolic and diastolic hypertension. Auscultation for midline abdominal or flank bruits should be part of every patient evaluation, especially if the patient presents with abrupt onset of flank pain, hematuria, or rapidly progressive hypertension, because there may be a renal artery dissection leading to renal infarction. Lower extremity edema, shortness of breath, or dyspnea on exertion may be indicative of volume overload due or congestive heart failure.
Reasons for referring a patient for a renovascular duplex evaluation include sudden onset of hypertension, abdominal bruits, uncontrolled hypertension in spite of a multiple drug antihypertensive regimen, sacral edema or bilateral lower extremity edema not relieved with diuretics, elevated creatinine, and acute azotemia during treatment with angiotensin-converting enzyme inhibitors. Labropoulos and associates26 identified uncontrolled and controlled hypertension as the main reasons for referral to a university hospital vascular laboratory for renal duplex scanning.
MESENTERIC ARTERIAL DISEASE
Patients with a history of abdominal pain and weight loss may be referred to the vascular laboratory for evaluation of the mesenteric arteries. The finding of an abdominal bruit is another indication to examine the mesenteric arteries, along with the aorta and the renal and iliac arteries. It is commonly stated that symptoms of chronic mesenteric ischemia occur only when there is severe disease (>70% stenosis) in at least two of the three major mesenteric arteries (celiac, superior mesenteric, and inferior mesenteric). Furthermore, the superior mesenteric artery must be one of the involved vessels. The slow progression of atherosclerotic stenosis is such that collateral pathways usually have time to develop, so single-vessel mesenteric arterial disease rarely results in symptoms. In a population-based study using duplex ultrasound, 17.5% of a population over 65 years of age was found to have asymptomatic mesenteric artery stenosis.27 However, if there is a sudden, complete occlusion of the superior mesenteric artery only, or if there is a superior mesenteric artery stenosis combined with previously interrupted collateral pathways, visceral ischemia may occur. In a retrospective study to identify factors affecting outcomes after surgical revascularization for chronic mesenteric ischemia, Mell and coworkers found that acute-on-chronic symptoms were present in 26% of patients. Presenting symptoms in these patients included postprandial pain (91%), weight loss (69%), and diarrhea (25%).28
The underlying pathophysiology of chronic mesenteric ischemia is failure to achieve hyperemic postprandial intestinal blood flow. In normal individuals, superior mesenteric artery blood flow increases after eating, with the maximum increase occurring at 30 to 90 minutes and the time interval to peak flow varying with the size and composition of the meal. Undiagnosed chronic mesenteric ischemia results in nutritional depletion. Dr. Strandness stressed to his students that you will never see an obese patient with symptomatic chronic mesenteric ischemia. In fact, he told us about a 350-pound woman who presented to the vascular surgery clinic for treatment of a celiac artery stenosis. The reason for referral was abdominal pain due to chronic mesenteric ischemia. While obtaining the history, the patient related to Dr. Strandness that she developed abdominal pain after eating large amounts of bacon and beans. She had not had any weight loss but had actually gained over 100 pounds in the previous 5 years.
The typical patient with symptomatic chronic mesenteric ischemia is a cachectic, middle-aged woman with a heavy smoking history who presents with postprandial abdominal pain and unintentional weight loss. The pain is usually located in the midepigastric region, occasionally radiates through to the back, and is often described as either dull or colicky. Postprandial pain can last from 1 to 3 hours and may be associated with nausea, vomiting, or diarrhea. Patients avoid certain types of food or almost stop eating altogether, resulting in weight loss. This specific behavior pattern is sometimes described as “food fear.” In these patients, weight loss is due to inadequate nutritional intake rather than an absorption problem.
The Cardiovascular Health Study followed 533 participants for a mean of 6.5 years and found no statistically significant association between the presence of mesenteric artery stenosis and subsequent acute or chronic intestinal ischemia, gastrointestinal tract interventions, all-cause mortality, or cardiovascular events.29 Duplex ultrasound is ideal for evaluating patients with an asymptomatic abdominal bruit, with median arcuate ligament compression of the celiac artery, or with suspected chronic mesenteric ischemia. However, it is less helpful in patients presenting with acute abdominal symptoms, because they are more likely to have technical limitations to abdominal ultrasound related to bowel gas. Mesenteric duplex scanning is discussed in Chapter 23.
VENOUS THROMBOSIS
Normal venous blood flow can be disrupted by a thrombus or thromboembolus obstructing the vein, incompetent venous valves, compression by adjacent structures, or a reduction in the effectiveness of the pumping action of the surrounding muscles. Decreased venous blood flow leads to increased venous pressure, a subsequent increase in capillary hydrostatic pressure, net filtration of fluid out of the capillaries into the interstitial space, and edema. Edematous tissues cannot receive adequate nutrition from the blood and, consequently, are more susceptible to injury, ulceration, and infection.
Lower extremity superficial veins, such as the great saphenous, are thick-walled vessels that lie just under the skin. Deep veins are thin walled and have a less muscular medial layer. As discussed in Chapter 6, normal deep and superficial veins have valves that maintain unidirectional flow back toward the heart. The valves are located at the base of a segment that is expanded into a sinus. This arrangement allows the valve leaflets to open without coming into contact with the wall of the vein, permitting rapid closure when the blood starts to flow backward. Perforating veins have valves that allow one-way blood flow from the superficial system to the deep system.
Three factors, known as Virchow’s triad, are believed to play a significant role in the development of venous thrombosis, and these are stasis of blood, vessel wall injury, and altered blood coagulation. Venous stasis occurs when the flow rate is reduced and the blood remains in contact with the venous wall longer than normal. This happens in heart failure or shock and when skeletal muscle contraction is reduced, as in immobility, paralysis, or during anesthesia. Bedrest reduces blood flow in the legs by at least 50%.1 Damage to the intimal lining of blood vessels creates a site for thrombus formation. Direct injury to the veins can result from fractures, blunt or penetrating trauma, and chemical irritation from intravenous medications. Increased blood coagulability occurs in patients who have had anticoagulant medications abruptly withdrawn and those with malignancies. Oral contraceptive use and a growing list of inherited conditions are also associated with hypercoagulability.
The annual incidence of deep vein thrombosis (DVT) is 1:10,000 but increases to 1:100 in patients over 70 years of age.30 Upper extremity DVT accounts for approximately 4% of all cases of venous thromboembolic disease (see Chapter 20).31 Exact figures for the annual number of deaths from pulmonary emboli are difficult to obtain because they come largely from autopsy statistics; however, autopsy series of hospitalized patients have shown that pulmonary embolism is the cause of 4% to 11% of deaths and only 1 in 4 of these patients had recent surgery.32
Risk factors for DVT have been studied in both surgical and medical patients. The Sirius study used a case-control design to assess clinical risk factors for DVT in outpatients, of whom 77.7% were medical patients. The factors that emerged as associated with DVT in the medical population are consistent with previous studies and include a history of venous thromboembolism, chronic venous insufficiency, heart failure, cancer, trauma, infectious diseases, pregnancy, and obesity. Immobilization, standing in one spot for more than 6 hours per day, long-distance travel, and a history of three or more pregnancies also emerged as factors associated with DVT.32
In general, upper extremity DVT is not as prevalent as lower extremity DVT. However, it is common in patients with central venous catheters and in those with an underlying disease that causes hypercoagulability such as cancer. Internal trauma to the veins may result from pacemaker leads, chemotherapy ports, dialysis catheters, or parenteral nutrition lines. Primary upper extremity DVT, which occurs in the absence of the aforementioned risk factors, has been found in approximately 30% of documented cases.31 Martinelli and colleagues studied a series of patients with primary upper extremity DVT and found an adjusted odds ratio for upper extremity DVT of 6.2 for factor V Leiden, 5.0 for prothrombin G20210A, and 4.9 for anticoagulant protein deficiencies.31 In women with factor V Leiden or prothrombin G20210A who were taking oral contraceptives, the odds ratio for upper extremity DVT increased to 13.6. In this same study, strenuous muscular effort with the affected arm was a common predisposing condition and was present in one-fourth of the patients.
Effort thrombosis is a disorder of the thoracic inlet in which chronic venous compression from repetitive overhead arm motion is thought to be the inciting factor leading to intimal damage and subsequent thrombosis. This is most likely to be seen in athletes such as swimmers, tennis players, and baseball pitchers. When subclavian-axillary vein thrombosis occurs, the arm becomes painful and swollen, and prominent superficial collateral veins may be visible over the shoulder area.33 Dr. Strandness had numerous athletic patients with effort thrombosis. One such patient was an avid tennis player, a sport that Dr. Strandness also enjoyed and played several mornings every week before coming to the hospital. So it is not surprising that he found it extremely difficult to counsel the patient to give up a sport they both loved. However, the patient began taking golf lessons and found that it did not produce the same upper extremity discomfort caused by tennis. This patient eventually became as passionate about golf as he had been about tennis, and at each clinic visit, he would challenge Dr. Strandness to a game. It became quite the running argument between the two of them as to which sport required the most skill.
A major challenge associated with diagnosing lower extremity DVT is that the signs and symptoms are nonspecific. Despite this variability, clinical signs should always be investigated. Obstruction of the deep veins results in edema or swelling of the extremity because the outflow of venous blood is impeded. There may be limb discomfort, a feeling of heaviness, and functional impairment. In some instances, there may be an increase in the temperature of the limb, but this is extremely variable. Although Homans’ sign (pain in the calf with passive dorsiflexion of the foot) has been used historically to assess for DVT, it is not a reliable or valid sign and has no clinical value in the diagnosis of lower extremity DVT. This was one of Dr. Strandness’ pet peeves, and he made sure every medical student knew that most patients with leg symptoms from any cause will complain of pain when you sharply dorsiflex their foot. In only one situation is the clinical presentation, by itself, adequate to arrive at a diagnosis of lower extremity DVT. This is with iliofemoral venous thrombosis (phlegmasia cerulea dolens), which involves extensive thrombosis of the common iliac, external iliac, common femoral, and femoral veins (and sometimes, the inferior vena cava). When this occurs, the entire limb becomes acutely swollen and cyanotic with severe pain. No other disorder produces this dramatic clinical picture.
External compression of the left common iliac vein is frequently seen in the general population and can result in obstruction of venous outflow from the left leg and contribute to the development of DVT. When a patient presents with chronic left lower extremity edema, pain, varicosities, and acute DVT with no predisposing risk factors, external compression of the left common iliac vein may be present. This is known as May-Thurner syndrome or iliac compression syndrome and is due to the right common iliac artery compressing the left common iliac vein against the fifth lumbar vertebra, causing chronic injury and intimal fibrosis, spurs, and webs.34
Thrombosis of superficial veins produces pain, tenderness, redness, and warmth in the involved area—a condition referred to as superficial thrombophlebitis. These patients usually present with an erythematous, palpable cord. Dr. Strandness always emphasized the point that inflammation does not play a significant role in deep vein thrombosis, so the term “thrombophlebitis” should only be used to describe this combination of thrombosis and inflammation in the superficial veins. The risk of superficial venous thrombi becoming dislodged or fragmenting into clinically significant emboli is very low.
Postthrombotic Syndrome
One of every three to four patients with symptomatic lower extremity DVT will develop postthrombotic sequelae.35 Chronic venous insufficiency can occur many years after an episode of venous thromboembolism, and up to 10% of patients develop leg ulcers.36 In a population-based, case-control study in New Zealand, Walker and associates found that people with a history of DVT were almost three times more likely to have a leg ulcer and that the risk of developing a leg ulcer was more than twice as likely in people who were at high risk for venous thromboembolism.36
Venous insufficiency results from a combination of obstruction of the deep veins in the legs and reflux of blood through incompetent valves. This leads to failure of the calf musculovenous pump and ambulatory venous hypertension (see Chapters 6 and 21). Because the veins are relatively large, thin-walled structures, they distend readily when the venous pressure is elevated. In this state, the leaflets of the venous valves are stretched and prevented from closing completely, allowing reflux of blood back toward the periphery. This persistently elevated deep venous pressure eventually causes the signs and symptoms of the postthrombotic syndrome.
The clinical presentation may include brownish skin discoloration (hyperpigmentation), subcutaneous fibrosis (brawny induration), venous ectasia, pruritus, leg heaviness, and swelling. Severe cases can also show intractable edema and skin ulceration. The patient may be less symptomatic in the morning and more symptomatic in the evening after the legs have been dependent all day. The swelling always involves the ankle area and may extend further up the limb, depending on the extent of the venous obstruction and valvular incompetence. However, if the edema also involves the dorsum of the foot, the clinical picture is more consistent with lymphedema. This is noteworthy because it is common to have patients referred to the vascular laboratory for venous studies when the underlying etiology is actually lymphatic obstruction. The region of hyperpigmentation is typically on the distal medial side of the calf and ankle, where the major perforating veins are found, but the ankle may be involved circumferentially (Fig. 1.7). The same pertains to the location of venous ulcers when they occur. The precise mechanism of venous stasis ulceration remains controversial, although it is generally accepted that chronic venous hypertension and incompetent perforating veins play a role.37 Postthrombotic skin changes develop as a result of the rupture of small veins in the skin or extravasation of blood components through enlarged capillary pores. Red blood cells escape into the surrounding tissues and then break down, leaving brownish hemosiderin pigment. The skin becomes dry and cracked, and the subcutaneous tissues fibrose and atrophy. Venous ulcerations are typically large but shallow with irregular edges and located in the “gaiter area” of the medial or lateral malleoli (Fig. 1.8).
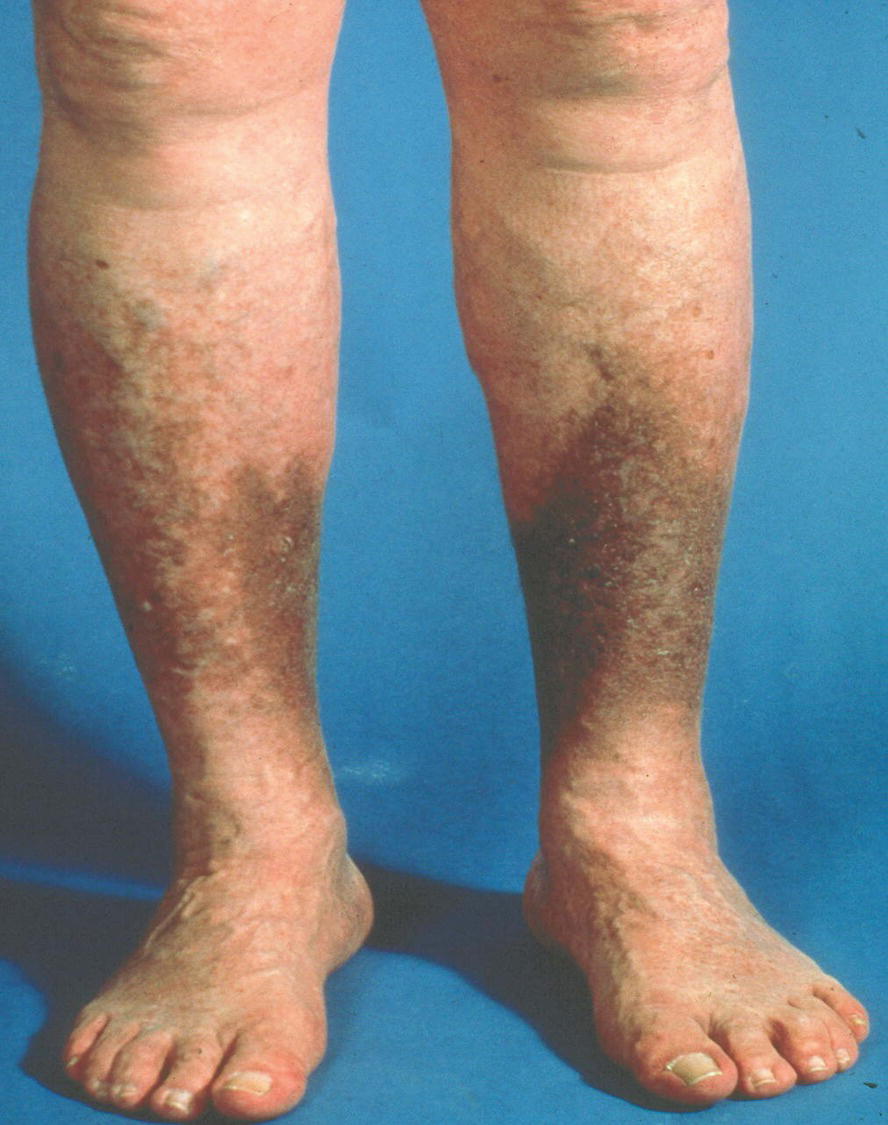
FIGURE 1.7. Hyperpigmentation in the “gaiter area” due to chronic venous insufficiency.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


