Fig. 15.1
Some common symptoms of multiple sclerosis
Other vascular problems of the cerebrospinal system produce different symptoms. Acute dural sinus or jugular vein obstruction, such as that caused by hypercoagulability, catheterization complication, or compression (tumor or lymphadenopathy), can cause acute symptoms of mental confusion, severe headaches, and visual disturbances. Treatment with angioplasty, with or without stenting, is often clinically successful [5]. Transient global amnesia has been hypothesized to be caused by internal jugular vein reflux [6]. CCSVI has not been found in association with other neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis [1].
CCSVI is distinct from venous sinus thrombosis, which is a well-established cause of acute mental status change, headache, and stroke. Venous sinus thrombosis may be caused by hypercoagulability, catheterization complications, or compression by tumor. The mainstay of treatment is systemic anticoagulation, but interventional techniques including catheter-directed lysis, mechanical thrombectomy, and angioplasty have been sporadically reported.
15.1.3 Anatomy and Physiology
Intracranial blood passes through the dural sinuses into the extracranial system of the internal jugular and (IJV) vertebral veins (Fig. 15.2). Most blood volume drains anteriorly through the IJV in the supine position and posteriorly through the vertebral veins in the standing position. The vertebral system also communicates with deep thoracic and lumbar and hemiazygos veins. The vertebral, deep thoracic and lumbar, and hemiazygos veins all drain into the final collecting azygos vein (AV).
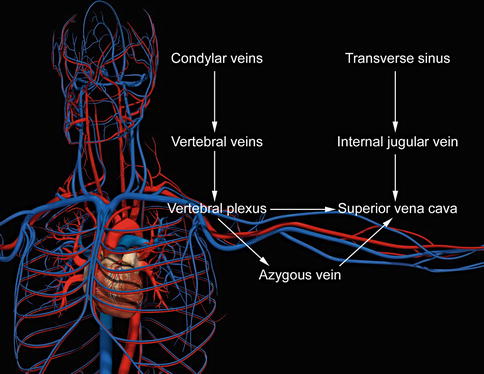

Fig. 15.2
Anatomy
The IJV and AV drain into the superior vena cava (SVC). Most CCSVI abnormalities occur near the junction at either the IJV or AV with the SVC and usually near at or near a valve. Physiologic obstructions also occur, such as at the skull base, adjacent to the carotid bulb, and where the strap muscles compress the vein [5]. Physiologic obstructions must be separated from pathologic obstructions, since the former should not be treated.
15.1.4 Pathophysiology
The classic pathophysiologic model of multiple sclerosis is that of an autoimmune disorder [7]. CCSVI advocates largely do not challenge the importance of this model in understanding the disease. MS plaques, however, also show impressive pathophysiologic similarities to chronic venous insufficiency of the lower extremities. Both show perivenous iron deposition and pericapillary fibrin cuffs. Activated macrophages show hemosiderin deposits and ferritin-like structures. There is hyperactivation of metalloproteinases and hypoactivation of tissue inhibitors of metalloproteinases [8].
15.1.5 Diagnosis
Duplex ultrasound has been proposed as a screening test for CCSVI. Key ultrasound findings are summarized in Fig. 15.3. Zamboni and colleagues have defined the details of the protocol. In this protocol, two or more of the five ultrasound criteria in Fig. 15.1 are considered positive for CCSVI [3]. The use of a different ultrasound protocol was ineffective in differentiating MS patients from controls [9]. The use of ultrasound to screen for CCSVI is training and protocol dependent [1].
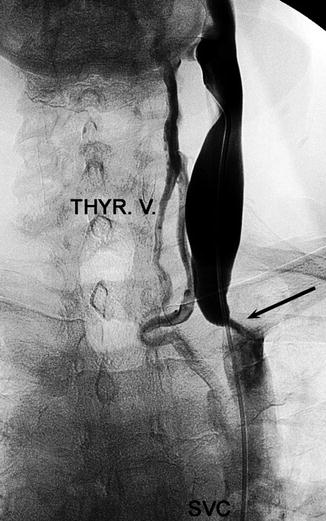

Fig. 15.3
Venogram showing venous obstruction (arrow) (Courtesy of Roberto Galleoti, University of Ferrara, Italy)
Venography is currently the primary test used to confirm CCSVI (Fig. 15.4) [10]. Common findings include, among others, annulus, septum malformation, or membranous obstruction. Magnetic resonance and computerized tomography venography as well as intravascular ultrasound have also been considered [1, 5].
15.1.6 Treatment
Angioplasty and stenting have been proposed as treatments for CCSVI. Treatment with angioplasty is being performed at specialized centers with good technical success. Stenosis recurrence is a problem, especially in the internal jugular veins [10]. Deep venous thrombosis and vein rupture have been rare complications [11]. Stent placement has also been performed, but there has been a case of stent migration reported [11, 12].
15.1.7 Conclusions
It is presently highly controversial whether CCSVI plays a clinically significant role in MS and whether fixing these venous obstructions will help MS patients. Clinical outcomes are currently the subject of an ongoing randomized controlled trial in Italy. The Society of Interventional Radiology Foundation recommends further study [13]. It is an important area of research, because it carries the potential to help a significant number of patients with a severely disabling disease at minimal risk.
15.2 Venous Thoracic Outlet Syndrome
15.2.1 Etiology
The etiology of subclavian vein obstruction may be primary, when there is no known reason for the obstruction, or secondary, in which there is a known reason for the obstruction to occur. In both primary and secondary subclavian venous obstructions, extrinsic pressure or intrinsic trauma can produce either a thrombotic or non-thrombotic occlusion secondary to stenosis of the subclavian vein.
A thrombus must be treated separately prior to further intervention to relieve the cause of the obstruction. The majority of patients have secondary subclavian vein obstruction from intimal damage due to the insertion of catheters or pacemaker wires.
Other known secondary causes are thrombosis from underlying coagulopathies, extrinsic pressure on the subclavian vein due to cancer, and from irradiation (which can cause intimal damage from ongoing vasculitis or extrinsic compression from scarring and fibrosis).
15.2.2 Pathophysiology
Primary subclavian vein obstruction is also known as effort thrombosis or Paget-Schrötter syndrome, which was first described by Paget in 1875 and von Schrötter in 1884. The underlying cause of primary subclavian vein occlusion is often due to a congenitally narrowed costoclavicular space (also termed the thoracic outlet) for passage of the subclavian vein as it joins the innominate vein. In the costoclavicular space, the costoclavicular ligament and subclavius muscle surround the subclavian vein as it passes between the first rib and the clavicle to enter the mediastinum.
The possible causes for primary obstruction of the subclavian vein are (1) enlargement of either the ligament or the muscle, (2) a narrow angle between the clavicle and the first rib, or (3) the position of the subclavian vein that is too medial compared to normal. In any of these possibilities, the vein lies too close to the costoclavicular ligament and is subject to trauma, particularly from strenuous arm motion, hence the rise of the term “effort thrombosis” to describe this condition. The repetitive trauma leads to intimal injury, thickening, or web formation, and stenosis can result. Thrombosis is the final event, and it may be acute or chronic or never occur.
15.2.3 Symptoms
Clinically, two-thirds of reported cases of subclavian vein thrombosis occur on the right side. This may be due to the acute angle between the right subclavian and innominate veins when compared to the left, which is almost straight, resulting in hemodynamically more turbulent flow on the right. Another proposed explanation is that more people are right-hand dominant and therefore the right arm is more likely to be used for strenuous activities. Men are more likely than women to develop subclavian vein obstruction, and the exact reason for this is still unknown. Paget-Schrötter syndrome is most often a disease of young, active, healthy patients.
Symptoms are the same for both thrombotic and non-thrombotic occlusions, and these include sudden swelling of the hand and arm, a pressure sensation of the arm, and pain, all of which are aggravated by physical activity. Some patients may describe the arm as having a “bursting” feeling. The majority of patients with non-thrombotic occlusions will have had a gradual onset of symptoms, while patients with thrombotic occlusions may have had an acute or gradual onset. In retrospect, many people with an acute thrombotic presentation often had earlier milder symptoms of pain and swelling but did not initially seek medical attention until more severe symptoms suddenly appeared. Patients who present after the initial venous thrombosis has resolved may only demonstrate symptoms with physical activity.
15.2.4 Diagnosis
On physical exam, in addition to the swelling of the hand and arm, there may be cyanosis or rubor and distended veins around the shoulder or lateral chest, indicating the development of collateral circulation (“first rib collaterals”). In patients with effort thrombosis, pallor, sweating, and fatigue may also accompany their hand and arm symptoms. Workup often starts with noninvasive duplex scanning, but occasionally it may not be possible to visualize the subclavian vein due to the clavicle. A positive duplex scan is followed by diagnostic venogram, which is the gold standard for diagnosis. If there is partial obstruction, dynamic venography is essential, as occlusions may not be seen unless the arm is elevated to 90–180°, hyperabducted, or even adducted [39]. See Chap. 9 for a further discussion of workup and diagnostic imaging.
15.2.5 Treatment
Secondary subclavian venous thrombosis is usually treated conservatively with anticoagulation: heparin initially followed by warfarin for 3–6 months. The offending indwelling catheters or wires should be removed. In dialysis patients, where their functioning arteriovenous fistula (AVF) is in the offending arm, removal of the AVF will often relieve the symptoms. However, if retention of the AVF is necessary, transluminal angioplasty (with stent placement if absolutely required) or surgical bypass via axillary, brachial-internal jugular bypass, or central vein bypass may be performed to decompress the arm.
Primary subclavian vein obstruction is usually symptomatic when presented and must be treated aggressively in the following order: (1) remove the acute thrombus if present and reestablish axillosubclavian venous patency, (2) relieve the extrinsic pressure by decompression of the costoclavicular space, and (3) eliminate the intrinsic defect. The acute thrombus is treated by catheter-directed thrombolysis with tissue plasminogen activator (tPA), urokinase (UK), or potentially, in some cases, by pharmacomechanical thrombolysis, followed by systemic anticoagulation to maintain venous patency with heparin followed by warfarin. Lytic management of acute venous thoracic outlet syndrome (TOS) is demonstrated in Fig. 15.5. Although thrombolysis is most successful in thrombus less than a few days old, it can dissolve clot several weeks to (in some cases) several months old. Indications for surgical thrombectomy are failure of lysis to reestablish venous outflow, patients who have contraindications to fibrinolytic therapy, or technical inability to deliver the agent directly into the thrombus of patients who experience persistence of severe symptoms (Fig. 15.6).
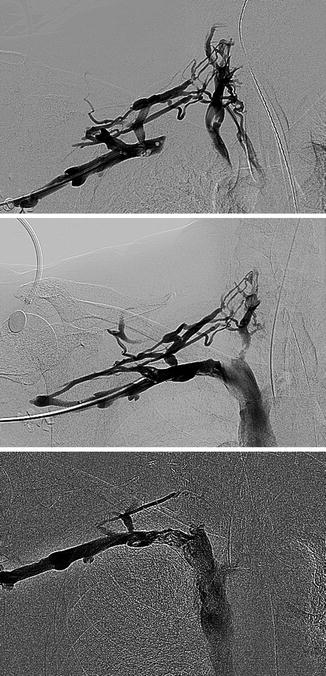
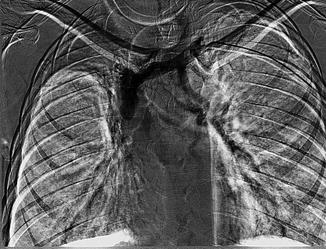

Fig. 15.5
Venous thoracic outlet syndrome

Fig. 15.6
Subclavian venous thrombosis
Once venous patency is established, the underlying cause of the occlusion should be repaired, and in most cases, this is due to the extrinsic compression of the subclavian vein at the costoclavicular ligament. The relief of extrinsic compression is by first rib resection, either by a transaxillary, supraclavicular, or infraclavicular approach. The supra- or infraclavicular approach may be optimal if concomitant exploration or reconstruction of the subclavian vein is anticipated. In any case, it is necessary that the anterior portion of the first rib be removed along with sufficient costal cartilage to totally free the subclavian vein.
The timing of resection of the first rib remains controversial. Traditional protocols advocated systemic anticoagulation for 3 months prior to surgical intervention, due to potential coagulation issues in the patient following lysis. Most surgeons believe there is no difference in rethrombosis of the vein despite a 3-month delay in surgery for extrinsic compression. However, currently in many centers, first rib resection is performed either during the same hospitalization or at the time of thrombectomy [39]. Rethrombosis of the vein following lysis or decompression should be treated with repeat lysis. If the subclavian vein cannot ultimately be opened by lysis or other techniques, some would omit first rib resection since there is no reason to decompress an already occluded vein, perhaps with the exception of an open proximal subclavian vein from a cephalic vein collateral. However, some argue that there is a potential role for first rib resection or other TOS surgery even in those with an occluded subclavian vein [28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



