Patients with only one ventricle (i.e., “functional single ventricle,” or the functionally univentricular heart) comprise a very heterogeneous group. With the Fontan operation as the preferred definitive palliation in patients with a univentricular heart, it remains important to determine if a given ventricular chamber is inadequate for support of either systemic or pulmonary circulation. Often, the atrioventricular (AV) connection is the determining factor.
Nomenclature and classification of the univentricular heart have long been subjects of debate and controversy, with terms such as “single ventricle,” “univentricular heart,” “univentricular atrioventricular connection,” and “double-inlet ventricle” being used over the years. Van Praagh and colleagues originally defined a univentricular heart as one ventricular chamber that receives both tricuspid and mitral valves or a common AV valve; hearts with one absent AV valve (including mitral and tricuspid atresia) were not included in this original review. Van Praagh et al. also pointed out that although these patients have a functionally univentricular heart, there are usually two ventricular chambers; thus, a “true” univentricular heart is exceedingly rare. Anderson and colleagues introduced the term “univentricular atrioventricular connection” and applied this to hearts where the AV connection was committed to one ventricle. They subsequently proposed that “univentricular heart of left ventricular type” be applied to hearts where the dominant ventricle was a morphologic left ventricle (LV) and “univentricular heart of right ventricular type” be applied to the dominant right ventricle (RV). Further characterization of the ventricular mass as consisting of three regions (inlet, trabecular, and outlet regions) was included. Assessment of a ventricle in this way could aid in the determination of the adequacy of a given ventricle, that is, whether a ventricle was complete or incomplete. However, a hypoplastic ventricle with all three components present does exist. In more recent publications on nomenclature, Jacobs and Anderson simply refer to the “functionally univentricular heart,” wherein the emphasis was placed on the inadequacy of one or the other ventricle to support the pulmonary or systemic circulation. Regardless of the preferred nomenclature, a segmental approach to echocardiographic evaluation is necessary in all cases, defining connections and relationships to provide the clinician with relevant information.
This chapter will cover the three main types of univentricular AV connection that produce a functionally univentricular heart: tricuspid atresia, mitral atresia (or hypoplastic left heart syndrome), and double-inlet LV (DILV). A brief review of the hypoplastic LV or mitral stenosis in the setting of multiple left-sided obstructive lesions is included. Typically, all of these conditions are the result of an absent, hypoplastic, or atretic AV connection.
All patients with a functionally univentricular heart require careful anatomic assessment to plan for a staged surgical approach that will provide definitive palliation. Although nomenclature may be debated, it is important that there is consensus regarding such nomenclature at the institutional level so that clinicians understand each other and are able to communicate clearly about the nature of complex congenital heart disease. A complete description of segmental anatomy and physiology, which inherently lends itself to clinical application and decision making, is most useful for the broadest audience.
TRICUSPID ATRESIA
Tricuspid atresia is the third most common form of cyanotic congenital heart disease, with a prevalence of 0.3% to 3.7%, and is characterized by absence of a direct communication between the right atrium (RA) and the RV (Fig. 12.1). There is a univentricular AV connection with the dominant ventricle having left ventricular morphology. The anatomic form of atresia is most commonly fibromuscular; less commonly, it is membranous, valvar, or Ebstein-like with valvar atresia. In the majority of patients, the floor of the RA is entirely muscular with separation from the hypoplastic RV by fibrofatty tissue. Although we use the term “tricuspid atresia” for lesions with an echogenic plate-like area beneath the floor of the right atrium and above the right ventricle, this echogenicity is usually not the result of an atretic tricuspid valve but rather it results from fibrofatty tissue in the AV groove. Therefore, tricuspid atresia is likely a result of failure of formation of the tricuspid valve rather than fusion of formed tricuspid valve leaflets. True atresia of tricuspid valve leaflets as seen in the atretic tricuspid valve in Ebstein anomaly is rare.
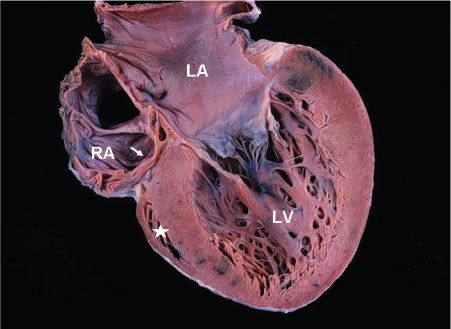
Figure 12.1. Pathologic specimen in tricuspid atresia showing the atretic fibrofatty tricuspid valve (arrow). The left atrium (LA) and left ventricle (LV) are enlarged. The right ventricle is extremely hypoplastic and appears as a “slit-like” space (asterisk). RA, right atrium.
Historically, based on the great artery relationship, tricuspid atresia is classified into three types, with subclassification based on the anatomy of the ventricular septal defect (VSD) and pulmonary valve (Table 12.1). However, to avoid miscommunication, the echocardiographer should describe the anatomy and physiology in detail.
Associated Anomalies
An opening in the atrial septum, either a patent foramen ovale or a secundum atrial septal defect (ASD), is obligatory for survival. Occasionally, the atrial septum is restrictive. Rarely, a primum ASD may be present. Thirty percent of patients with tricuspid atresia will have additional associated cardiac anomalies, including left superior vena cava (SVC) (16%), juxtaposed atrial appendages (more common with transposed great arteries), and coarctation of the aorta (8%). Associated cardiovascular anomalies are more common with transposed great arteries (63%), compared with normally related great arteries (18%). Also, approximately 20% of patients will have extracardiac anomalies, including gastrointestinal and neurologic defects.
Clinical History
The majority of patients with tricuspid atresia present with cyanosis. Patients with tricuspid atresia and normally related great arteries (ventriculoarterial concordance) have a high incidence of subvalvular or valvular pulmonary stenosis (less commonly, atresia). Clinical presentation depends on the amount of pulmonary blood flow, which is proportional to the size of the VSD and the degree of pulmonary valvar/subvalvular stenosis. If there is no significant pulmonary stenosis or restriction of the VSD, these patients may present between 4 and 8 weeks of age with signs and symptoms of pulmonary overcirculation (similar to the infant with a large VSD) and only mild hypoxemia due to the large amount of pulmonary blood flow. If not recognized early, these infants are at risk for longer-term complications from hypoxemic pulmonary overcirculation and elevated vascular resistance. In the presence of pulmonary atresia or critical pulmonary stenosis, closure of the ductus arteriosus results in severe cyanosis, hypoxemia, and acidosis, and, if not treated promptly, may result in death. Patients with transposed great arteries (ventriculoarterial discordance) typically have unobstructed pulmonary blood flow; as pulmonary vascular resistance drops in the neonatal period, these infants may also present with signs of congestive heart failure and pulmonary edema. However, if there is significant aortic arch obstruction or critical restriction of the VSD (supplying systemic output), once the ductus arteriosus closes, cardiovascular collapse and shock will develop.
Echocardiographic Examination of Tricuspid Atresia
Echocardiography in the neonate with tricuspid atresia provides comprehensive diagnostic information. Diagnostic cardiac catheterization is rarely needed. Careful attention to the absent right AV connection, the arrangement of the great arteries, the nature of the communication between the LV and hypoplastic RV, and the presence of aortic arch or pulmonary artery (PA) obstruction should provide the clinician with complete diagnostic assessment, allowing for accurate planning of staged surgical palliation.
Subcostal Views
Subcostal four-chamber (coronal) view Subcostal examination begins with a determination of abdominal viscera and atrial situs in all patients. Subcostal four-chamber (coronal) views will show dilation of the RA with absence of the connection to the RV (Fig. 12.2A [Video 12.1A]). As foreshortening of the RV may occur in this plane, short-axis (sagittal) plane imaging is useful for “three-dimensional” assessment of right ventricular size. The atrial septum is best visualized from subcostal imaging planes, and characterization of the ASD should be performed. Prominent Eustachian valve tissue may be present but typically does not contribute to obstruction. Color Doppler will show a right-to-left shunt from the RA to the left atrium (LA) and no flow from the RA to the RV. It is unusual for the ASD to be restrictive, but pulsed-wave Doppler interrogation should be used to evaluate the RA-to-LA gradient, tracing the signal over three cardiac cycles to determine a mean gradient. An atrial shunt is obligatory for survival; therefore, a restrictive ASD may result in severe hemodynamic compromise requiring urgent septostomy. Evaluation of the great arteries from multiple imaging planes to determine ventriculoarterial connections is important (Fig. 12.2B–C [Video 12.1B, C]). An enlarged, posterior great artery (PA) that bifurcates early is consistent with transposed great arteries (ventriculoarterial discordance) (Fig. 12.3A–B [Video 12.2A]). Examination of the ventricular septum may provide information on the size and location of the VSD, but orthogonal views will be needed. In tricuspid atresia, the VSD is usually muscular; rarely, the VSD can be doubly committed and subarterial or outlet in nature. The mitral valve and left ventricular function can be assessed initially from the four-chamber subcostal plane.
A left-juxtaposed right atrial appendage is visualized in the subcostal four-chamber scan plane. Both atrial appendages are located more leftward than normal. Echocardiographers should be alert to a left-juxtaposed right atrial appendage when visualizing an abnormal convexity of the atrial septum to the left or a transverse orientation of the septum when imaging posteriorly from the subcostal four-chamber plane. Further anterior angulation of the probe will reveal the connection of the RA to the leftward right atrial appendage. Juxtaposition of the appendages should not be confused with an ASD.
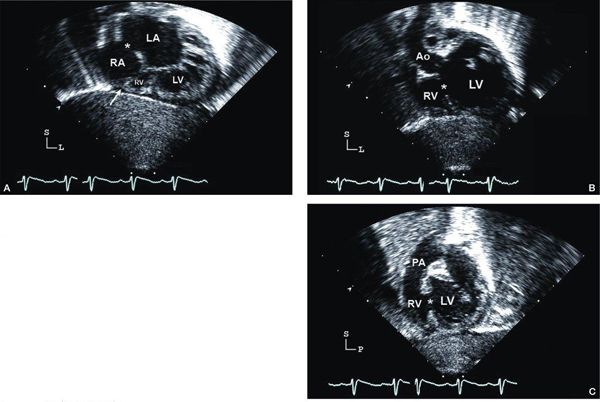
Figure 12.2. Tricuspid atresia with normally related great arteries; subcostal views. A: “Four-chamber” (coronal) view showing moderate-sized secundum atrial septal defect (asterisk), atretic tricuspid valve (arrow), hypoplastic right ventricle (RV), and dilated left ventricle (LV). B: Four-chamber view, angled anteriorly, illustrating the large ventricular septal defect (asterisk). Aortic (Ao) origin from LV in normally related great arteries. C: Short-axis (sagittal) view showing anterior RV, posterior LV, and muscular ventricular septal defect (asterisk). In normally related great arteries, the pulmonary artery (PA) arises anteriorly from the RV and bifurcates early. LA, left atrium; RA, right atrium (Video 12.1).
Subcostal short-axis (sagittal) view Subcostal short-axis views demonstrate the absent connection between the floor of the RA and the hypoplastic RV. Orthogonal views are very useful for evaluation of the atrial septal anatomy. Again, the right-to-left shunt should be unrestricted in the setting of an adequate interatrial communication. Rightward angulation of the transducer facilitates evaluation of the absent communication between the RA and the hypoplastic RV, and the size of the RV is more easily assessed in the subcostal short-axis view than in the four-chamber imaging plane (Figs. 12.2C and 3C–D). Evaluation of the size of the VSD between the LV and hypoplastic anterior RV is important for documenting sites of obstruction to arterial outflow. Careful sweeps from right to left are important to obtain complete information about the location and degree of right ventricular outflow obstruction. Assessment of the ventriculoarterial connection is performed from the short-axis view; again, the proximal bifurcation of the PA should be assessed. The presence of parallel great arteries suggests transposition (ventriculoarterial discordance). A small anterior aorta should prompt a careful evaluation for coarctation of the aorta from additional views.
Parasternal Views
Parasternal long axis Parasternal long-axis scans typically demonstrate a small anterior RV and a large posterior LV (Fig. 12.4A). This scan plane also provides excellent views of the ventricular septum. The size and position of the VSD should be noted (see Fig. 12.4A). The position and origin of the great arteries are confirmed. In the presence of transposed great arteries, the arteries appear parallel in their proximal course from the ventricles, with the posterior vessel (PA) bifurcating early (Fig. 12.5A). If the VSD is present in the outlet portion of the septum, anterior deviation of the septum (seen most commonly with normally related great arteries) may produce subpulmonary obstruction. Posterior deviation is seen more often with transposed great arteries. Muscular ridges or membranes can also cause ventricular outflow obstruction and should be evaluated from multiple imaging planes. Anterior angulation of the transducer toward the patient’s left shoulder may bring the right ventricular outflow tract (RVOT) into view. Doppler and color Doppler evaluation of the gradient from the LV to RV and into the RVOT should be used to provide information about the degree of restriction, either to the PA (for an estimation of the PA pressure) or to the anterior aorta in transposition.
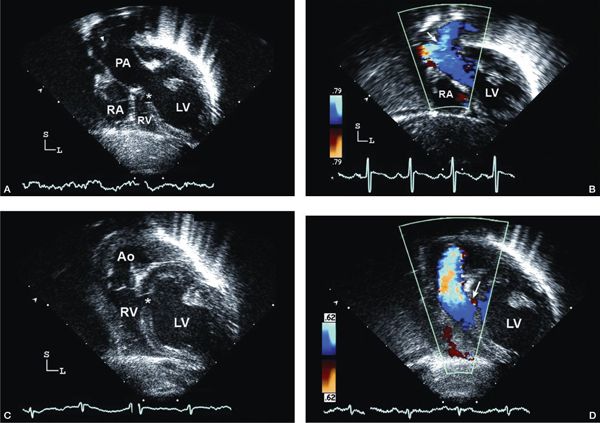
Figure 12.3. Tricuspid atresia with transposed great arteries; subcostal long-axis (coronal) views. A: Dilated left ventricle (LV), hypoplastic right ventricle (RV), and a small muscular ventricular septal defect (asterisk). Note the pulmonary artery (PA) arising from the LV with early bifurcation (arrowhead). B: Color Doppler imaging in the same patient demonstrating flow in the PA bifurcation (arrow). C: Slight anterior angulation of the transducer demonstrates the LV, hypoplastic RV, and the restrictive ventricular septal defect (asterisk). The anterior aorta (Ao) arises from the hypoplastic RV. D: Color Doppler demonstrating the flow across the small ventricular septal defect (arrow), antegrade into the aorta (Video 12.2).
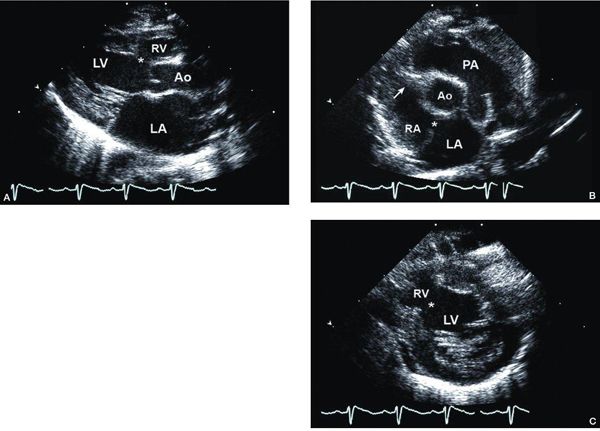
Figure 12.4. Tricuspid atresia with normally related great arteries; parasternal views. A: Long-axis view showing small anterior right ventricle (RV) and dilated posterior left ventricle (LV). There is a muscular ventricular septal defect (asterisk). B: Short-axis view at the cardiac base demonstrating normally related great arteries, an atretic tricuspid valve (arrow), and a secundum atrial septal defect (asterisk). C: Short-axis view at the level of the mitral valve (M) demonstrating the dilated LV communicating with the hypoplastic RV through a large muscular ventricular septal defect (asterisk). Ao, aorta; PA, pulmonary artery (Video 12.3).
Visualization of the atrial septum in a perpendicular orientation from the long-axis view may be consistent with a left-juxtaposed right atrial appendage. A dilated coronary sinus should alert the echocardiographer to the possibility of a left SVC returning to the coronary sinus (which has implications in planning for the bidirectional cavopulmonary shunt as palliation).
Parasternal short-axis view The parasternal short-axis view is useful for further characterization of the hypoplastic RV and VSD and position of the great arteries (Figs 12.4B and 5B). Left ventricular function should be evaluated. Scanning apically from the base of the heart toward the midventricular level, the right ventricle is seen in front of the dominant, large LV (Fig. 12.4C). In addition to orthogonal subcostal views, the size of the RV and the anatomy of the VSD can be assessed in the short-axis plane (Fig. 12.4C). The presence of additional VSDs should be assessed with both imaging and color Doppler. Pulsed-wave Doppler interrogation can provide an estimation of the gradient between the LV and RV as well as aid in assessing the restrictive VSD. Scanning superiorly toward the base of the heart to the level of the great arteries will again confirm their arrangement. If transposed, both great vessels are seen in short-axis, represented by two semilunar valves seen in the same imaging plane (Fig. 12.5B). In transposition, one can evaluate whether the anterior aorta is located rightward (d-transposition, more common) or leftward (l-transposition, less common).
It is necessary to evaluate the degree of restriction of the RVOT in the short-axis view. Color Doppler and pulsed-wave Doppler should be used in this assessment. With normally related great arteries, the presence of a large PA suggests that adequate or generous pulmonary blood flow is present. If the PA is very small or diminutive, it is necessary to confirm the presence of antegrade flow from the RV to the PA. Rarely, pulmonary atresia coexists with tricuspid atresia, creating a ductal-dependent critical condition. The ductus arteriosus should be assessed fully. Typically, a trifurcation view of the ductus, left PA, and right PA can be obtained in a high left parasternal plane.
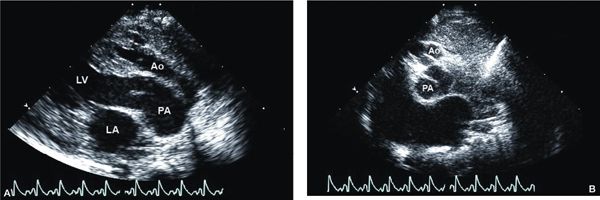
Figure 12.5. Tricuspid atresia with transposed great arteries; parasternal views. A: Long-axis view showing the parallel orientation of the great arteries classically seen in transposition, with an anterior aorta (Ao); posterior pulmonary artery (PA) originating from the dilated left ventricle (LV). B: Short-axis view at the cardiac base showing both semilunar valves in short-axis at the same level, again consistent with transposition of the great arteries. The aortic valve (Ao) is hypoplastic and located anterior and slightly rightward, whereas the pulmonary valve (PA) is dilated and located posterior and leftward. LA, left atrium (Video 12.4).
Apical Views
An apical four-chamber view provides excellent definition of the absent right AV connection (Fig. 12.6A [Video 12.5A]). Angling the transducer posteriorly demonstrates the muscular atresia of the tricuspid valve appearing as an echo-dense plate of tissue in the floor of the RA. Again, assessment of the hypoplastic RV and its communication from the LV (VSD) is important. In the presence of juxtaposed atrial appendages, one can again see an abnormal atrial septal configuration. Mitral valve morphology and function are well visualized from the apical approach. Angling the transducer anteriorly facilitates development of a “five-chamber view,” providing further assessment of the outflow tracts and sites of potential obstruction (Fig. 12.6B–C). The origin, size, and position of the great arteries and outflow tracts again are assessed in light of information obtained from all of the previous views. The size of the VSD and any evidence of obstruction are evaluated with both pulsed-wave and color Doppler, attempting to align the transducer beam in a parallel fashion with the VSD or outflow tracts. Para-apical imaging, directing the transducer more anteriorly toward the anterior great artery, can also facilitate assessment of obstruction and gradients.
Suprasternal Views
Beginning with the long-axis view of the aortic arch, careful evaluation for evidence of arch obstruction is very important. In the setting of transposition of the great arteries, coarctation is more common, particularly when the VSD is restrictive and the aorta is small (Fig. 12.7A–B [Video 12.6A]). Critical coarctation in the neonate requires immediate recognition so ductal patency can be maintained appropriately until surgical intervention can be performed. Doppler interrogation of the coarctation gradient is unreliable due to the ductal flow distal to the site of coarctation (Fig. 12.7C). In the setting of coarctation of the aorta, a large ductal arch is present (this can be visualized from multiple views, in addition to suprasternal imaging). The native, diminutive aorta may insert “end-on-side” onto the large ductal arch (see Fig. 12.7A). Typical Doppler interrogation of the ductus will show bidirectional shunting with right-to-left flow in systole and reversal of flow into the PA branches in diastole. The amount of left-to-right diastolic flow is proportional to the pulmonary vascular resistance, with more flow seen with lower resistance. In normally related great arteries, the ductus is usually longer and narrower, as fetal flow patterns are predominantly from the aorta into the PA. If there is critical obstruction to PA flow from the RV, the ductus may be quite tortuous.
Short-axis suprasternal imaging demonstrates the branching pattern of brachiocephalic arteries and the sidedness of the aortic arch. The PA bifurcation is also seen from short-axis imaging, with branch sizes measured. The absence of an innominate vein should raise the possibility of a left SVC. A typical “crab” view should demonstrate normal pulmonary venous connections.
Tricuspid atresia vs. Double-inlet left ventricle In both tricuspid atresia and double-inlet left ventricle, the dominant ventricular chamber is the left ventricle. However, in double-inlet left ventricle both AV valves are committed to the dominant left ventricle, either through two valves of which one may be atretic, or through a common AV valve, and the ventriculoarterial connection is commonly discordant. In tricuspid atresia, only the morphologic left atrium is connected to the dominant left ventricle and ventriculoarterial connection is most frequently concordant.
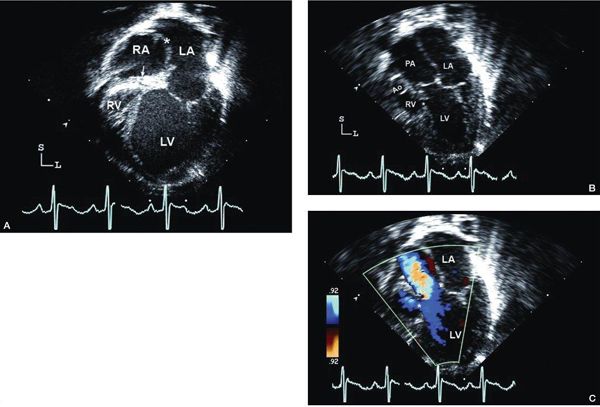
Figure 12.6. Tricuspid atresia with transposed great arteries; apical views in same patient in Figure 12.4. A: Four-chamber view demonstrating the dilated left ventricle (LV), atretic tricuspid valve (arrow), and severely hypoplastic right ventricle (RV). There is a secundum atrial septal defect (asterisk). B: Slight anterior angulation of the transducer from the four-chamber view shows the aorta (Ao) arising from the hypoplastic RV and the dilated pulmonary artery (PA) from the LV. C: Color Doppler imaging demonstrates antegrade flow into the PA with a small amount flow into the restrictive ventricular septal defect (asterisk). LA, left atrium (Video 12.5).
HYPOPLASTIC LEFT HEART SYNDROME
Hypoplastic left heart syndrome (HLHS) is the fourth most common congenital cardiac anomaly of infancy, with a reported prevalence of 0.016% to 0.036% of live births, and occurs twice as often in boys as in girls.
Anatomy
HLHS encompasses a heterogeneous group of cardiac malformations characterized by normally related great arteries and varying degrees of underdevelopment of the left heart–aorta complex, resulting in obstruction to systemic cardiac output and the inability of the left heart to support the systemic circulation. The spectrum of anomalies includes mitral stenosis (Fig. 12.8A) or atresia, hypoplasia of the LV, aortic stenosis or atresia (Fig. 12.8B), and hypoplastic aortic arch. At the most severe end of the spectrum are mitral and aortic atresia, with a severely hypoplastic or “slit-like” LV. In milder forms, there is hypoplasia of the aortic and mitral valves and a varying degree of left ventricular hypoplasia. The ventricular septum is usually intact; a VSD, if present, is usually small. In rare cases of a large VSD with mitral atresia, the LV is usually well developed.
In HLHS, the systemic circulation is dependent on the RV and the ductus arteriosus. The aortic arch, ascending aorta, and coronary arteries are perfused by retrograde flow. Coarctation of the aorta is typically present.
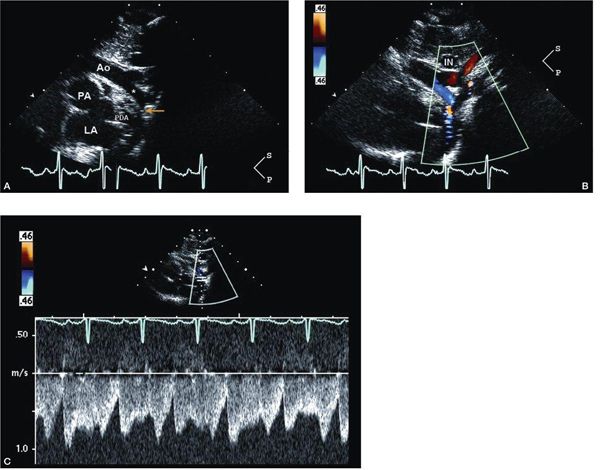
Figure 12.7. Tricuspid atresia with transposed great arteries and restrictive ventricular septal defect; suprasternal views. A: Long-axis (sagittal) view in the same patient described in Figures 12.4 and 12.5 showing the hypoplastic transverse aortic arch (asterisk) and coarctation of the aorta (yellow arrow). B: Color Doppler imaging shows antegrade flow in the area of coarctation. C: Pulsed-wave Doppler interrogation of the coarctation in the presence of a large patent ductus arteriosus shows low-velocity flow. Doppler in this setting is unreliable in predicting the true severity of coarctation. Two-dimensional anatomic assessment is critical. Ao, aorta; IN, innominate vein; LA, left atrium; PDA, patent ductus arteriosus (Video 12.6).
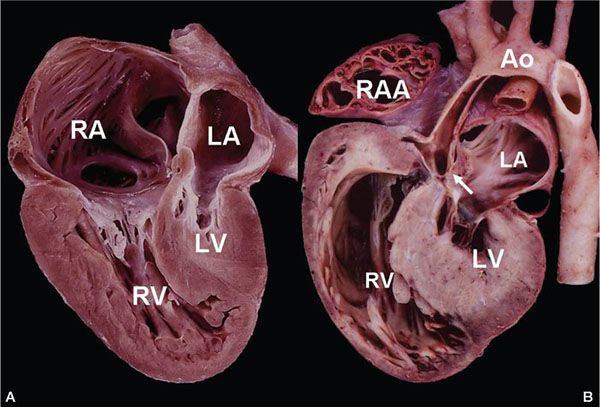
Figure 12.8. Pathologic specimen from a patient with hypoplastic left heart syndrome (HLHS). A: Four-chamber view showing severely hypoplastic left ventricle (LV), hypoplastic mitral valve, and a small left atrium (LA). The right ventricle (RV) and right atrium (RA) are dilated. B: Long-axis outflow view demonstrates the atretic aortic valve (arrow) and hypoplastic aorta (Ao).
The presence of an intact or highly restrictive atrial septum has been recognized as a predictor of poor outcome among patients with HLHS. An intact atrial septum is present in approximately 6% of patients with HLHS; however, clinically deleterious restriction to flow at the level of the atrial septum can occur in as many as 22% of patients. During fetal life, a severely restrictive atrial septum may be associated with nonimmune fetal hydrops and pulmonary lymphangiectasia. In the presence of an intact atrial septum and mitral atresia, the only egress of blood from the LA may be a levoatriocardinal vein, a pulmonary-systemic connection that provides an alternative route for pulmonary venous blood to enter the systemic venous circulation. In the majority of patients, this vein connects the LA to the innominate vein. However, it may drain to other sites, including the left SVC or jugular venous system.
Clinical Presentation
The majority of fetuses with HLHS tolerate this cardiac anomaly well, with the majority of infants born at full-term, initially healthy in appearance. However, acute hemodynamic collapse follows closure of the ductus arteriosus (which may occur after discharge from the newborn nursery). If ductal patency is not restored promptly, poor systemic perfusion results in hypoxemia, acidosis, shock, and eventual death. On examination, the infant appears poorly perfused, tachypneic, and pale, with diffusely diminished pulses. There may be only a nonspecific cardiac murmur, but an S3 gallop is very common. The second heart sound is loud and single. There is often hepatomegaly. Echocardiographic identification of HLHS should prompt immediate intervention with infusion of prostaglandin E1 to maintain or improve ductal patency.
Infants with HLHS and a nearly intact atrial septum present with severe pulmonary venous hypertension and are even more critically ill with severe respiratory distress. These patients often present with cardiogenic shock and profound cyanosis at birth, needing immediate catheter-based septostomy/cutting balloon dilation of the atrial septum for survival to a palliative surgical procedure.
Echocardiography in Hypoplastic Left Heart Syndrome
The overall approach to imaging in HLHS is to provide complete diagnostic and hemodynamic information. Cardiac catheterization is reserved only for the patient requiring emergent intervention (usually for enlargement of the ASD). An assessment of right ventricular and tricuspid valve function, ductal physiology, and atrial septal anatomy is crucial for clinical management.
Systemic and pulmonary flow ratios are dependent on the difference in resistance between the two respective vascular beds. A large, nonrestrictive interatrial communication in the setting of low pulmonary resistance promotes preferential flow into the pulmonary vascular bed at the expense of the systemic circulation. Pulmonary overcirculation and imbalance in the pulmonary/systemic vascular resistance ratio contribute to hemodynamic instability in infants with HLHS. Conversely, a restrictive ASD in the presence of severely elevated PA pressure and resistance produces preferential right-to-left ductal shunting and restricted pulmonary blood flow at the expense of the patient’s oxygenation. Intervention to relieve severe obstruction may be needed before definitive surgical palliation. Thus, in patients with HLHS, the clinical presentation can be variable. Immediate echocardiographic assessment of the underlying physiology is critical for management of the patient before palliative surgery is planned and undertaken.
Subcostal Four-Chamber (Coronal) View
The subcostal four-chamber view typically demonstrates a dilated RA and RV. When angling the transducer posteriorly and imaging toward the base of the LA, the LV either appears very small or is not visualized (Fig. 12.9A [Video 12.7]). It should be immediately apparent when the LV is diminutive or “slit-like” that a significant discrepancy in the size of the RV compared with the LV is present. Once this view is obtained, the echocardiographer should evaluate the mitral valve and the great arteries very carefully. Anterior angulation of the transducer typically shows the dilated RVOT and main PA (MPA), but the very tiny aorta may be difficult to visualize in coronal plane imaging. A large patent ductus arteriosus (PDA) may be seen, essentially representing a continuation of the MPA as the ductal arch, but this is better visualized in subcostal short-axis views. In the setting of cardiovascular collapse, right ventricular function may also be reduced, sometimes significantly so. Tricuspid regurgitation is usually present in this clinical setting.
The atrial septum should be carefully evaluated from the subcostal windows. The size, number, and location of communications from LA to RA should be assessed (Fig. 12.9A–B). Bulging of the atrial septum from the LA into the RA is suggestive of restriction to egress from the LA. Also, in the presence of a restrictive or intact atrial septum, the atrial septum is usually thick and the pulmonary veins are dilated (if normally connected). Color Doppler aids in mapping of the defects with the shunt typically occurring from left to right (see Fig. 12.9B). Alignment of the Doppler cursor parallel with the defect allows estimation of the mean transeptal gradient by tracing the Doppler signal across three cardiac cycles.
Bidirectional shunting is very unusual but may be seen in the presence of severe tricuspid regurgitation or anomalous venous connections. The echocardiographer should be alert to the possibility of anomalous connection and drainage of the pulmonary veins. While present in a minority of patients with HLHS, this should be suspected particularly if venous return into the diminutive LA cannot be seen from subcostal imaging.
Subcostal Short-Axis (Sagittal) Views
Orthogonal plane imaging provides confirmation of the cardiac anatomy. Subcostal short-axis views are excellent for interrogation of the atrial septum. The presence/location of atrial communications should be determined and color Doppler mapping of atrial shunting should be performed. The relative size of the very hypoplastic aorta posteriorly and dilated PA anteriorly is evaluated. Again, by angling the transducer rightward, the continuation of the dilated MPA as the ductal arch is easily demonstrated. Color Doppler interrogation may show bidirectional PDA shunting (typically right-to-left in systole with left-to-right shunting in diastole depending on pulmonary resistance characteristics). The entire aortic arch may be visualized in this view (with definitive imaging obtained from suprasternal imaging). Scanning toward the midventricular level shows the enlarged anterior RV and hypoplastic, posterior LV (Fig. 12.9C). Right ventricular function and tricuspid regurgitation should be assessed.
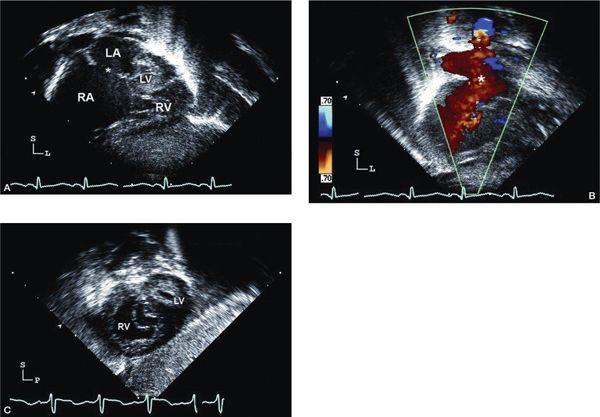
Figure 12.9. Hypoplastic left heart syndrome (HLHS); subcostal views. A: Long-axis (coronal) image showing very dilated right atrium (RA) and small left atrium. The secundum atrial septal defect (asterisk) is large and unrestrictive. The right ventricle (RV) is hypertrophied, and the left ventricle (LV) and mitral valve are severely hypoplastic (Video 12.7). B: Color Doppler imaging demonstrating nonrestrictive laminar flow across the atrial septal defect (asterisk). C: Short-axis (sagittal) view in the same patient, angled toward the midventricular level showing the anterior, hypertrophied RV. Note the echo-bright LV endocardium often seen in HLHS.
Parasternal Views
Parasternal long-axis view Parasternal long-axis views confirm the size discrepancy between the large RV anteriorly and the diminutive or small LV posteriorly (Fig. 12.10A). Careful examination for the slit-like, muscle-bound LV confirms that the anterior ventricle is the RV. The ventricular septum is most often intact. Right ventricular systolic function can be evaluated from the long-axis view, although angling the transducer inferiorly toward the tricuspid inflow is usually required. Left ventricular systolic function is usually severely decreased if a cavity is present. In this instance, the left ventricular endocardium and papillary muscles may be echo-bright, suggesting the presence of endocardial fibroelastosis secondary to long-term subendocardial ischemia. Mitral and aortic valve leaflets should be examined for mobility or patency. The mitral annulus is characteristically hypoplastic with the mitral valve and its subvalvular apparatus appearing abnormal. If patent, the valve may be thickened and doming, with shortened chordae. A supravalvar mitral ring may also be present. The aortic valve is usually completely atretic but may be thickened and doming. Subaortic obstruction may be present. The size of the hypoplastic aortic annulus and ascending aorta (usually an internal diameter of less than 5 mm) is more easily measured in the parasternal long-axis view (see Fig. 12.10A).
The transducer is angled anteriorly and superiorly to evaluate the RVOT, the dilated MPA, and the ductal arch. Typically, the ductus is very large. With Doppler interrogation, the PDA shunt is right-to-left during systole, while the amount of left-to-right shunt during diastole is dependent on the pulmonary vascular resistance. Moving the transducer to a high left parasternal window may bring the large ductus/ductal arch into view more easily. Pulsed-wave Doppler is used to evaluate a ductal gradient in the setting of early ductal constriction as typically indicated by an increased systolic Doppler flow velocity through the PDA (> 2.5 m/s).
From parasternal long-axis imaging, the LA is usually small. However, the LA can be dilated in the presence of a nearly intact atrial septum.
Parasternal short-axis view In the parasternal short-axis view, there is a large anterior RV and small posterior LV (Fig. 12.10B). This view is also useful in the assessment of ventricular function, mitral valve size and morphology, and mitral papillary apparatus number and position. The mitral valve may be “parachute-like” in nature with a single papillary muscle group. Short-axis scans at the base of the heart allow assessment of the ascending aortic size in cross section and further evaluation of aortic valve morphology. The great arteries are normally related with the severely hypoplastic aorta in the center. The coronary artery origins are most often normal and may appear as extensions of the diminutive aorta (Fig. 12.10C). The MPA is usually much dilated and the PDA is prominent (see Fig. 12.10C). The branch PAs should be assessed in this view, with slightly higher positioning of the probe on the patient’s chest to obtain the trifurcation view (see Fig. 12.10C). Again, with color Doppler and pulsed-wave Doppler examination, the PDA flow is most typically bidirectional. A systolic gradient should be assessed for early ductal constriction (particularly if the prostaglandin infusion has not been initiated).
Apical Views
An apical four-chamber view provides comparison of the relative sizes of the ventricles. The RV is typically dilated and hypertrophied. The LV is small, muscle bound, and non-apex forming (Fig. 12.11A [Video 12.9]). The mitral valve annulus is usually hypoplastic with either an atretic opening or severely stenotic leaflets. If flow is present, one should assess the degree of stenosis from this view (taking into account that a larger interatrial communication may reduce the measured gradient). Furthermore, the mitral leaflet excursion may be limited in the presence of critical aortic stenosis or atresia, secondary to severely elevated left ventricular end-diastolic pressure. The transmitral gradient may also be artificially reduced in this setting. Spectral and color Doppler flow may be used to evaluate mitral inflow, mitral regurgitation, and aortic outflow (if the valve is patent). Tricuspid valve function should be assessed carefully; significant tricuspid regurgitation is a poor prognostic indicator (Fig. 12.11B–C). Anterior angulation of the probe to a para-apical view will show the dilated MPA, and color Doppler assessment of pulmonary valve regurgitation should be performed.
Suprasternal Views
Suprasternal long-axis scans provide an excellent view of the ascending aorta, aortic arch, and upper descending aorta. The ascending aortic size may range from mild to severely hypoplastic (Fig. 12.12 [Video 12.10]); however, the caliber of the aorta is much larger at the level of the innominate artery and beyond. Coarctation of the aorta is usually present (Fig. 12.13A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


