Fig. 3.1
A 21-year-old with HIV infection. (a) Chest CT scan with diffuse nodular disease suggestive of miliary tuberculosis. (b) Transbronchial biopsy specimen revealing caseating granuloma. (c) Ziehl–Neelsen stain revealing acid-fast bacilli
The technique, safety, and complications (e.g., pneumothorax, hemorrhage, transient pyrexia, and transient dyspnea) of TBB are beyond the scope of this chapter and are reviewed in detail by Tagliaferro et al. [11] The precautions to be kept in mind, however, are that only one lung should be sampled in order to avoid the occurrence of bilateral pneumothorax or hemorrhage [12]; it is also recommended that patients be hospitalized overnight following the procedure [11].
In a recent review article on childhood bronchoscopy [3], mention is made of new applications and techniques that are being introduced to the pediatric bronchoscopy practice such as endobronchial ultrasound and transbronchial needle biopsy of lymph nodes. The potential uses of endobronchial ultrasound in pediatrics was recently reviewed [13]. It is a minimally invasive technique that allows tissue sampling of peripheral lung lesions or mediastinal/hilar masses with high diagnostic accuracy and significantly lower morbidity and mortality compared to alternative approaches. Radial probe endobronchial ultrasound is used in adults for the investigation of peripheral lung lesions and could be adopted in children to achieve accurate biopsy of such lesions. Linear probe endobronchial ultrasound allows minimally invasive biopsy of mediastinal and hilar lesions.
Ultrasound-guided transbronchial needle aspiration (TBNA) of mediastinal lymph nodes is widely used in adults for cancer diagnosis [14]. This technique is not commonly utilized in pediatric patients; however, successful use of the minimally invasive technique of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was reported when sarcoidosis was diagnosed via material from hilar adenopathy in a 13-year-old child [15]. Given the size of the EBUS bronchoscope, application to younger children is not feasible; the largest report of pediatric TBNA for mediastinal lymphadenopathy did not use EBUS [16]. In this prospective study of 28 children (median age 41 months; range 9–168 months) guidance to the site of the biopsy was based on presence of enlarged subcarinal lymph nodes on chest CT scan reconstruction and the visual appearance of the carina. Definitive diagnosis by TBNA was found in 54 % of cases and in 36 % of the cases, cytology performed in the bronchoscopy suite led to the diagnoses. The authors concluded that TBNA is a safe procedure that adds value to flexible bronchoscopy in the diagnosis of mediastinal lymphadenopathy in children.
The limitations discussed above in regards to TBB for the diagnosis of parenchymal disease, predominantly in ILD, led to recent successful introduction of transbronchial lung biopsy by flexible cryo-probe. The technique allows acquisition of large biopsy samples of lung parenchyma that exceed the size and quality of samples obtained by forceps biopsy [17]. No pediatric reports are yet available, but in an adult study comparing historical controls to transbronchial cryo-biopsy in lung transplantation patients, no significant bleeding or pneumothorax occurred following transbronchial cryo-biopsy. The mean duration of bronchoscopy using cryo-probe was significantly shorter than the traditional forceps biopsy technique (5 vs. 8 min, respectively). The mean diameter of the specimen taken by forceps in historical controls was 2 mm compared to 10 mm obtained using the cryo-probe with no crush artifacts observed; ultimately, overall improved diagnostic value was reported [18].
Bronchoscopy for Removal of Obstructive, Noxious, or Damaging Materials from the Airway or the Lung
Bronchoscopy, both rigid and flexible, has been used for removal of various endogenous and exogenous materials in the airways that interfere with gas flow or exchange. This segment will cover in detail foreign body aspiration and also touch on less common conditions.
Bronchoscopy for Aspirated Foreign Bodies in Children
Removal of foreign bodies (FB) is by far the most common procedural challenge for the bronchoscopist. The US Centers for Disease Control (CDC) report almost 200,000 accidents per year resulting in nonfatal injury from foreign body aspiration in children less than 10 years of age [19]. These numbers may be even larger in other parts of the world, with a recent report from Algeria, where the authors state: “Foreign body aspiration is a real public health problem in Algeria” [20].
The decision about the need for intervention for suspected FB was addressed in a retrospective study of 160 children [21] aimed at exploring the best clinical and radiological predictors for finding a FB via bronchoscopy. Foreign body aspiration (FBA) was proven bronchoscopically in 122 (76 %). In multivariate analyses independent predictors of FBA were focal hyperinflation on chest radiograph, witnessed choking, and white blood cell count greater than 10,000/mL. Once there is suspicion of FBA, Martinot [22] proposed a management algorithm to assist in the decision between flexible versus rigid bronchoscopy based on the experience with 83 children. The authors propose rigid bronchoscopy to be performed first in case of asphyxia, a radiopaque FB, or association of unilaterally decreased breath sounds and obstructive emphysema. In any other case, flexible bronchoscopy is to be performed first for diagnostic purposes. They comment that if the algorithm was applied retrospectively to the 83 children in their study, it would have decreased the negative first rigid bronchoscopy rate to 4 %. They concluded that flexible bronchoscopy was a safe and cost-saving diagnostic procedure in children with suspected FB aspiration.
Rigid rather than flexible bronchoscopy has been advocated as the preferred instrument for extraction of foreign bodies since the early days of pediatric bronchoscopy [23] and continues to be the predominant practice [20]. Age appears to be a factor in decision-making. To this end, a study involving 102 infants (mean age 10.5 months, the youngest being 2 months old) with FBA, rigid bronchoscopy was used exclusively with a high success rate [24].
While the value of flexible bronchoscopy, as pointed out by Martinot et al. [22] is now widely accepted, the conventional teaching on extraction of aspirated FB points to the primacy of rigid over flexible bronchoscopy for such procedures because of its obvious advantages for visualization and instrumentation. It is conceivable, however, that this ongoing preference that emerges in literature is colored by fear of litigation if not abiding by “conventional” practice. This may create a distorted impression of limited value for flexible bronchoscopy. For the purist amongst the readers a “Cochranian” settlement of the question cannot emerge from literature that lacks any attempt for a controlled approach, neither is it likely that such evidence will emerge. The following segment attempts to provide experience on the role of flexible bronchoscopy for FBA.
Successful use of flexible bronchoscopy for extraction of FB has emerged over time, and some authors prefer flexible scopes for FBA. In a review of the Mayo Clinic Pediatric experience (1990–2001) [25] the authors preferentially used flexible bronchoscopy for extraction of FB in children. In their experience the procedure was successful and safe in children who underwent the procedure. The authors advise however that provisions be made to secure immediate rigid bronchoscope availability should the flexible bronchoscopic procedure be unsuccessful. Encouraged by previous reports of success and motivated by local circumstances or availability of relative expertise in flexible bronchoscopy, another publication [26] espoused flexible bronchoscopy for FBA. While this recommendation may be appropriate for some environments, our own experience is that we do not perform flexible bronchoscopy for suspected FB until immediate availability of rigid bronchoscopy is secured, along the lines of Swanson et al. [25].
A valid, and likely incontrovertible indication for preference of flexible over rigid bronchoscopy is FB that is lodged distally, beyond the reach of the rigid bronchoscope. Such conditions clearly justify an attempt of extraction with the more maneuverable flexible scope, yet may pose unique challenges as a result of angulation and depth of penetration into the bronchial tree with ever decreasing bronchial diameters as more distal branches are involved. Figure 3.2 depicts successful forceps extraction of a pin from a distal airway via flexible scope.
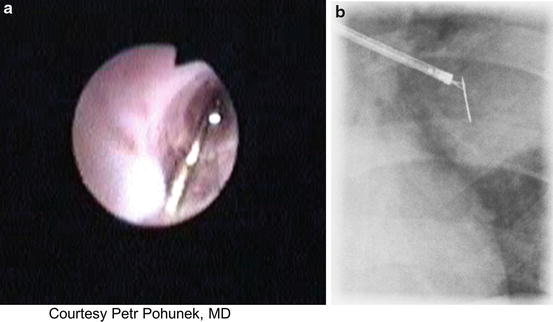

Fig. 3.2
Flexible bronchoscopic extraction of a foreign body (pin) in a 10-year-old. (a) Bronchoscopic image of the pin within the airway lumen. (b) Fluoroscopic image of bronchoscopic extraction of the pin. (Courtesy: Petr Pohunek, MD. Prague, Czech Republic)
An important complicating factor can be posed by a FB that is imbedded in the surrounding tissue, often a granulation reaction, rendering the object invisible. Such circumstances may lead to surgical intervention and resection of the involved segment. Two reports however address such conditions with the use of interim procedures or techniques in an attempt to loosen the embedded foreign body, in both cases an aspirated tooth, followed by successful extraction by using urologic baskets, balloon catheters or by forceps [27, 28]. These techniques included the use of topical and parenteral steroids and the use of argon plasma coagulation. The authors did not comment on late outcomes or complications of the interim procedures. The comment to make however is that these reports potentially understate the risk of dislodgement of the FB, which is often the argument cited to act with minimal delay when suspected.
With peripheral location of the FB or with bronchial lumina that are narrow in pediatric patients, visual limitation can complicate use of the flexible scope and instrumentation for extraction of a distal FB. This occurs when forceps passed through the working channel obstructs the field of vision within the narrow bronchus. Two studies describe the use of fluoroscopy [29] and image intensifier [30] to guide the grasping forceps for extraction of FB embedded in tissue past direct vision.
Sedation/Anesthesia for Foreign Body Associated Bronchoscopy
A detailed discussion on the sedation/anesthesia elements of flexible bronchoscopy is beyond the scope of this chapter. We will limit the comments on the topic to state that diagnostic procedures are mostly done through a nasal route, and recently often via laryngeal mask airway (LMA). A study of 1,947 procedures spanning the years 1988–2003 preferred use of LMA for flexible bronchoscopy in children 2 years of age and older, and complication rates were lower with the LMA (1.9 %) compared to the nasal route (3.5 %) [31].
In the context of this segment on FBA, while LMA is unlikely to be the choice approach when FBA is suspected, its use was reported in five cases in which FB was an incidental finding during a routine procedure and removed without difficulty and without the need to switch from the LMA to the conventional endotracheal tube [32].
Cast/Plastic Bronchitis
Cast or plastic bronchitis is a disorder characterized by formation of tenacious casts within the tracheobronchial tree. Spontaneously coughed up casts can draw attention to this uncommon condition. The distribution can be patchy or involving central segments of the airways when casts assume the shape of bronchial branching. Severe and sometimes life threatening obstruction can result. A comprehensive review of the topic is offered by Madsen et al. [33]. The underlying mechanisms involved in formation of casts are varied and overall not well understood; however, children with asthma who have particularly tenacious secretions may be affected and often improve with aggressive asthma therapies. At risk, albeit uncommonly, are children with various congenital heart disease, and in particular those who undergo Fontan procedures. A variety of therapies, all anecdotal, have been suggested for the cardiac-related conditions. It has been claimed that Bronchoscopy for removal of casts that obstruct large airways can be lifesaving but our experience has often found it difficult and extremely time-consuming due to the gelatinous consistency of the deposited material that renders suctioning, lavage, or removal by forceps difficult or unsuccessful.
Pulmonary Alveolar Proteinosis
Pulmonary Alveolar Proteinosis (PAP) is a rare pediatric disorder consisting of accumulation of phospholipid-proteinaceous material in the alveoli. Primary variety generally presents in infancy and early childhood and the acquired variety manifests in the older age groups. The underlying pathology is related to abnormal surfactant homeostasis and predominantly to defects in GM-CSF signaling. Shah et al. [34] and Mallory [35] comprehensively reviewed the topic. High resolution chest computerized tomographic scans (HRCT) with “crazy paving” patterns and flexible bronchoscopy with bronchoalveolar lavage are typically the key to the diagnosis when it yields milky fluid from affected segments with the extracellular substance staining with PAS [36]. The cytology is dominated by foamy macrophages [34, 35]. The most commonly considered procedure for a therapeutic intervention for PAP is a whole lung which is both challenging and time consuming. There are a number of approaches to the placement of the endotracheal tube (ETT) and isolation of the lung that is to be lavaged [37, 38]; The role of bronchoscopy is to secure the placement of the ETT and positioning of the balloon to prevent overflow of fluid into the ventilating lung. In essence a balloon catheter is placed in one main bronchus to seal off the entire lung that is to be lavaged with large amounts of saline, while ventilation is entirely dependent on the contralateral bronchus and lung. In exceptional cases where whole lung lavage is not feasible, a more arduous approach is that of direct segmental or subsegmental BAL [39, 40].
Lipoid Pneumonia
An extension of the concept of lung lavage was reported by Ciravegna et al. [41], in a case of an 8-year-old diagnosed with exogenous lipoid pneumonia due to aspiration of mineral oil that was administered for constipation. The diagnosis was supported by CT scan and BAL fluid (BALF) that was milky-appearing, yielding a high number of lipid-laden alveolar macrophages, as well as diffuse, free droplets of oil between alveolar cells on histology. Lung lavage was performed in the affected segments resulting in rapid clinical and radiologic improvement. A broader experience for the same diagnosis was reported in a study of 10 children with lipoid pneumonia secondary to mineral oil aspiration [42]. The authors took a stepwise BAL approach, which resulted in overall favorable outcomes.
Other Exogenous Foreign Material Aspiration
Sand aspiration to the lung in a 3-year-old with near-drowning was reported [43]. BAL was done when the child continued to have persistent wheezing and high ventilatory requirement and sand was detected in the BALF. Sequential lung washing followed by exogenous surfactant led to rapid improvement and subsequent recovery in PFTs. In a reported case of accidental instillation of activated charcoal into the lung by a misplaced gastric tube [44], an attempt was made to lavage the charcoal from the lung. While charcoal particles were observed in the BALF, the therapeutic effect could not be assessed since the case was complicated by severe pleural involvement.
Management of the Narrowed or Obstructed Airway: Debridement, Dilation, and Stenting
Impingement on airway lumen by tissue projecting into the lumen can result from various types of mechanical irritants and inflammatory processes both likely compounded by infection. Mechanical irritants and inflammatory processes in the airway lumen can both produce granulation tissues which with or without secondary infection can cause impingement on the airway lumen. Granulation tissue can follow irritation caused by an aspirated foreign bodies, endotracheal tubes, tracheostomy cannulas, and at surgical sites. These conditions can be approached by debridement using the forceps via flexible bronchoscope, compression by high-pressure balloon catheters and ultimately laser photoresection.
An example of granulation tissue following bronchial anastomosis in lung transplantation being treated using gentle excision by forceps via flexible bronchoscopy is presented in Fig. 3.3. Caution should be exercised since bleeding may be a complication and use of laser therapies that offer various option should be considered [1]. Soong et al. [45] described successful treatment of obstructive fibrinous tracheal pseudomembranes complicating central airways in 8 children following prolonged intubation using a combination of forceps, balloon and laser. Flexible bronchoscopic breaching, debridement, and dilation of what was assumed to be inflammation-related obstructive membranes was described in patients with CF and other post-infectious or inflammatory lesions [46, 47].
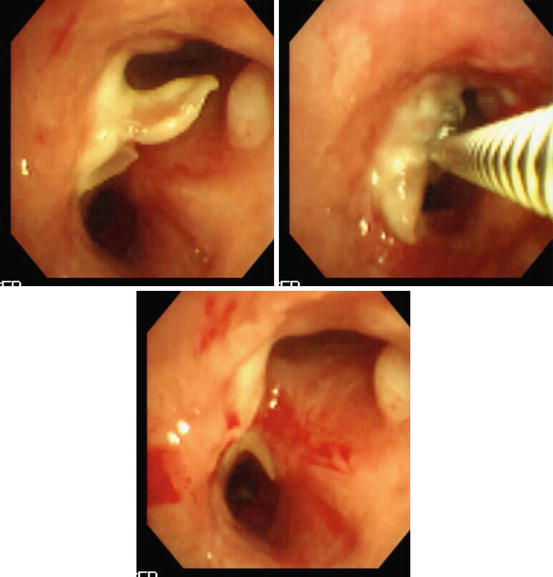

Fig. 3.3
Forceps debridement of postsurgical (anastomotic region after lung transplantation) scar tissue. The lower image shows patency of the airway lumen after the procedure
The use of balloon dilation for airways can be considered for a variety of conditions both congenital and acquired in which the airway wall is narrowed [48, 49]. The procedure can be done under bronchoscopic vision, but a radiologic approach has also been proposed [48]. An example of a bronchoscopic view of balloon dilation is presented in Fig. 3.4. Such pathology may recur and eventually may require stent placement. Importantly, balloon dilation can be considered for congenital narrowing of central airways such as complete tracheal rings [50]. This procedure should however be viewed as a surgical intervention since the risk of laceration and fracture of tracheal rings would require extreme caution. While evaluation of long-term outcome of balloon dilatation in adults is published, the indications and conditions of the procedure are so widely different from those in pediatric patients such that it appears unreasonable to extrapolate the results. Thus, little information about the long-term results in pediatric patients is available [51].
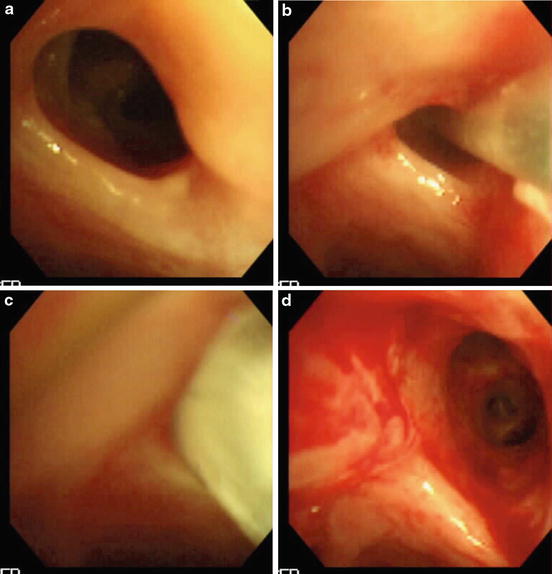

Fig. 3.4
(a–d) Improved patency is gained by high-pressure balloon dilation (c depicts balloon in place) in the narrowed bronchial anastomotic segment of a patient who underwent lung transplantation
Placement of Stents in the Airway
Over the past two decades there has been a growing body of information on the use of tracheo-bronchial stenting in pediatrics that has slowly gained recognition as an acceptable technique for the treatment of central airway obstruction, however, there [52]. Stents though an attractive proposition continue to be a topic of discussion and debate in the pediatric pulmonary practice as their limitations generally render them unready for prime time.
Stenting of the airway has been used successfully in adults, and has been considered as an attractive alternative in children. Fundamental differences of pediatric compared to adult use include the benign nature of most stenoses which do not alter life expectancy, [52] the narrow and soft airways of children, that improve with airway growth and the shift of mediastinal vessels [53] and also the required long-term tolerance and adaptation to growth. These differences may significantly alter the therapeutic balance, calling into question the precise role stents play in the treatment of airway obstruction in children. However, recognition that situations exist in which no other options are available has led to increased use of this technique in pediatrics.
Obstruction of the airway is the result of abnormalities of the airway wall, intraluminal causes or extrinsic compression [48]. Prior to stent placement, the airway is typically evaluated with bronchoscopy or bronchography and the chest evaluated for causes of compression with echocardiogram, CT scan or MRI [48].
Stents may be placed by either interventional bronchoscopists or invasive radiologists with relative advantages and disadvantages to each, and often cooperation between them. Plastic/silicon stents were initially available. The first use of a metallic stent in pediatrics was reported in 1988 [54]. Currently biodegradable stents are being introduced and offer potential new horizons [55]. The advantages and disadvantages of the various stents are further discussed below.
Most reports of use of stents in pediatrics are case reports or small series. In the absence of randomized clinical trials or larger series, it is difficult to compare the efficacy and tolerance of metal versus silicone airway stents in children; furthermore mortality rates in recipients of stents are generally high given that indications for stenting are mostly options of last resort [53].
Indications
The indications for stent placement as outlined in the adult literature include: extrinsic stenosis of central airways with or without intraluminal components due to malignant or benign disorders; complex, inoperable tracheobronchial strictures, tracheobronchial malacia, palliation of recurrent intraluminal tumor growth, and central airway fistulae (esophagus, mediastinum, pleura) [56]. In pediatrics the common causes of obstruction leading to stenting are the following:
Congenital stenosis. This results from abnormal cartilage rings (small and complete), or compression by abnormal vessels, such as pulmonary artery slings. Operative repair is the standard of care, however, recurrent obstruction is often encountered, the result of malacia or restenosis. Balloon dilations and stenting is thereafter sometimes the next step [48]. It may also be the result of accumulation of metabolic products such as seen in mucopolysaccharide storage disorders [57, 58]. It is usually agreed that in cases involving vascular compression, relief of the compression is the initial step [58].
Tracheal or bronchial malacia. This usually resolves by 2 years of age; however, it may require intervention when diffuse and/or requiring treatment with long-term CPAP via tracheostomy [48]. In a series of 105 patients who underwent aortopexy for treatment of tracheo-bronchomalacia, five patients required stenting after failure of aortopexy [59]. Additionally, despite the generally favorable long term prognosis of airway malacia, severe “dying spells” [60, 61], or severe growth retardation [58] may require a temporizing procedure.
Airway obstruction at a site of previous surgery. This usually results from granulation tissue and/or fibrosis following patch repair or over suture lines [62]. This condition may occur after lung transplantation at the site of the bronchial anastomoses.
Palliative indications. Stenting is also considered for palliation. This includes patients in whom a lesion is unresectable because of anatomic constraints, metastatic disease or limitations due to overall medical condition; stent placement may be minimally invasive and may provide prolonged palliation [63]. Stenting may allow weaning of ventilatory support and subsequently allow hospital discharge, even if long term survival is not anticipated [48].
Types of Stents
There are several different types of stents with their respective advantages and disadvantages, different methods of insertion, and varying requirements for follow-up and management.
A plethora of stents have been used in the airways. They can be divided into four major groups.
1.
Polymer stents (Dumon, Polyflex)
2.
Mettalic stents Balloon expandable (Palmaz) Self-expanding (Wallstent)
3.
Covered Mettalic stents
4.
Hybrid Stents
Stents can also be grouped based on indication, insertion technique, anatomical location or whether removable or not [64]. In 2011 use of a biodegradable polydioxanone stent was first reported in children [55].
Silicone or silastic stents are long tubes that are easy to remove but have problems with luminal occlusion and to a lesser degree migration [48]. The small radii of airways of children require thin walled stents, and when made of silicon these tend to be collapsible and prone to migration [58]. The continuous, non-fenestrated tube interrupts mucociliary clearance for which humidification and inhalation, inhalation of, mucolytic agents including DNase have been suggested, but their efficacy has never been documented [58]. Insertion is with a rigid bronchoscope, a device most pediatric pulmonologist are not familiar with such that placement is done only in centers with specialists trained in this procedure. Fayon et al. reported their experience with a custom manufactured polysiloxane (Tracheobronxane) stent in 14 children with success and failure rates equal at 43 %; the latter due to migration or obstruction.
In summary, extreme caution is needed when using these stents that remain attractive mainly for short-term use in the hospital setting postoperatively. Stents with internal support structures in their walls (Polyflex) aim to resolve some of these problems; however, migration and mucous impaction remain significant and limit their use [58].
Metallic stents were initially developed for vascular lesions. They are relatively easy to deploy by bronchoscopy or bronchography, are thin walled and their mesh structure allows for continued mucociliary clearance and ventilation even when the stent covers bronchial openings [58]. Their main disadvantage is difficult removal as early as several weeks after implantation due to mucosal overgrowth [58] albeit removal has been documented up to 5 years after insertion [64]. Other problems include breaks due to material fatigue and migration into surrounding organs [58].
The most frequently used balloon expandable stent is the Palmaz stent. It is non-elastic and made of stainless steel. Its main advantage in pediatrics is the ability to overdilate as the child grows, while its disadvantages are fracture [48] or deformation with cough [58]. They have the advantage over the self-expanding stents (such as the Wallstent; see below) in that they do not exert constant outward pressure after placement, which is implicated in erosion and hemorrhage [58]. In a 5-year published experience with this stent; a total of 30 stents were placed via rigid bronchoscopy in 16 patients [62] with airway malacia as the most common indication. Repeat procedures were required in several patients due to obstruction, development of granulation tissue and migration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


