Fig. 11.1
Placement of sensors and variables collected during PSG
Frontal, central, and occipital EEG
R. outer canthus and L. outer canthus EOG
Chin EMG
R. and L. anterior tibia EMG
(g)
Body position, and presence or absence of snoring via a video camera and microphone.
Optional recordings available in some labs and/or by special request include transesophageal balloon manometry (records intrathoracic pressure swings and may be useful in the diagnosis of suspected SRBD with negative PSG findings, such as upper airway resistance syndrome (UARS)), extended EEG montage for nocturnal seizure assessment, and pH probe recording for evaluation of gastroesophageal reflux.
The PSG is divided into 30-s segments (“epochs”) (Fig. 11.2). The study is then divided into different stages according to the patient’s sleep stage. The latter is being determined on the basis of the EEG (brain activity), EOG (eye movement), and EMG (skeletal muscle tone). The sleep/wake stages are the following:
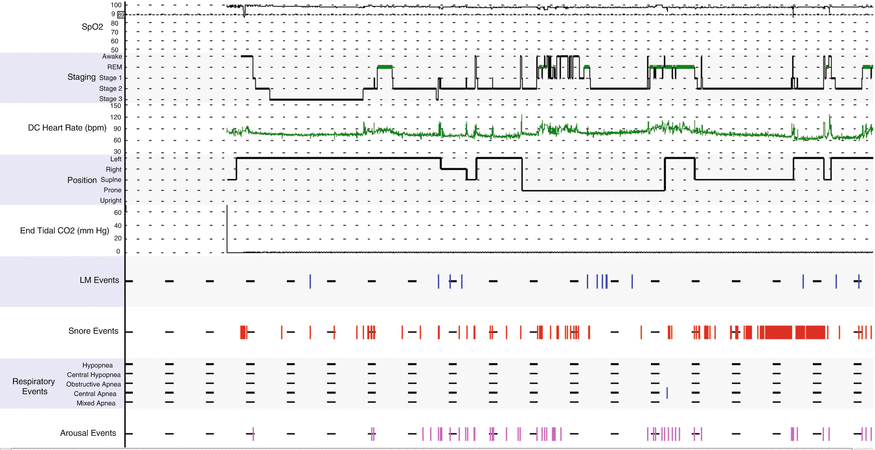

Fig. 11.2
Hypnogram shows mild sleep disruption, snoring, and increased arousals. REM sleep latency is prolonged. Leg movements are noted in stage II sleep. Oxygen saturation is normal. Only one central apnea event is noted during the latter portion of the night
1.
Wake or W
2.
Stage 1 or N1 (transitional sleep)
3.
Stage 2 or N2 (sleep characterized by specific EEG features—K complexes and sleep spindles)
4.
Stage 3 or N3 (combines prior stages 3 and 4; slow-wave, deep, or delta sleep)
5.
REM or stage R (rapid eye movement sleep)
The determination of the sleep stages allows the generation of several sleep-specific parameters which are reported as follows:
1.
Lights out/on
2.
Total recording time (TRT—lights out to lights on)
3.
Total sleep time (TST)
4.
Sleep latency (SL)—time to first epoch of sleep
5.
Stage R latency (sleep onset to first epoch of stage R)
6.
Wake after sleep onset (WASO)
7.
Sleep efficiency (SE) = TST/TRT × 100
8.
Time in each stage
9.
Percent of TST in each stage
10.
Arousals:
(a)
Total number
(b)
Arousal index (no. of arousals per hour of sleep time)
Respiratory parameters are determined by airflow (nasal/oral thermistor and nasal pressure transducer) and respiratory effort (RIP), as well as EEG arousals to assess sleep fragmentation associated with respiratory events:
1.
Number of apneas (obstructive, mixed, central), hypopneas, apneas + hypopneas
2.
Indices (index = number of events per hour of sleep): Apnea index (usually reported separately as central AI and obstructive AI), hypopnea index (HI), apnea + hypopnea index (AHI): This last parameter is the one most frequently used to determine the presence and severity of SRBD and is often subdivided into total (includes central events) and obstructive AHI.
3.
Optional:
(a)
Number of respiratory efforts related to arousals (RERAs) and RERA index (number of events/hour)
(b)
Paradoxical breathing (asynchronous chest and abdominal wall muscle movement indicating increased respiratory effort)
Oxygenation (pulse oximeter) and ventilatory status (end-tidal CO2 or transcutaneous CO2) parameters reported are the following:
1.
Number of oxygen desaturations >3 or 4 % from prior reading
2.
Oxygen desaturation index (number of desaturation episodes >3 %/h)
3.
Mean oxygen saturation
4.
Minimum oxygen saturation (and whether or not there is an association with any respiratory events)
5.
End-tidal carbon dioxide (optional in adult studies, but essential in pediatric studies)
Cardiac events as assessed by EKG:
1.
Average heart rate (HR)
2.
Maximum HR during recording, and during sleep
3.
Bradycardia, tachycardia, asystole, and other arrhythmias.
Movement events as assessed by bilateral EMG anterior tibialis monitors and EEG arousals:
1.
These can be limb movements or periodic limb movements (PLMS). Periodic limb movements are scored when there are four or more limb movements in succession with an interval that ranges from 5 to 90 s. The total numbers are reported for each one as well as the index (no. of events per hour of sleep).
2.
Number of PLMS with arousals as well as PLM arousal index (no. of events per hour of sleep).
The hypnogram (Fig. 11.2) is a visual display that gives an overview picture of the study night and sleep architecture, cardiorespiratory, and movement-related parameters.
Respiratory Indications for NPSG in Children
The indications for performing a PSG have been addressed in several recent publications by the American Academy of Sleep Medicine (AASM) in March 2011 and the American Academy of Pediatrics (AAP) in October 2012. A synopsis of the AASM diagnostic and treatment practice parameters is as follows (descending strength of evidence level is indicated by standard (S), guideline (G), and option (O)):
Diagnostic Indications (Children and Adolescents)
1.
Nocturnal in-lab PSG is a reliable and valid measure of the presence of OSAS.
(a)
Nap (abbreviated) PSG is not recommended for the evaluation of OSAS in children as it is likely to underestimate the presence and severity of SRBD (low sensitivity) (O).
(b)
There is currently insufficient data to support the use of unattended in-home portable PSG testing in the clinical diagnosis of SRBD in children. While there is an increasing trend towards the use of portable monitoring in adults with suspected SRBD, the feasibility and diagnostic accuracy of these devices in the pediatric population have yet to be determined.
2.
NPSG is indicated when the clinical assessment suggests the diagnosis of OSAS in children (S).
(a)
OSAS in children should be diagnosed based on clinical and PSG data.
(b)
The clinical evaluation (history and physical exam, audio/visual recordings, screening questionnaires, etc.) alone does not have sufficient sensitivity/specificity to establish the diagnosis.
3.
NPSG should be strongly considered if there is the slightest clinical suspicion of SRBD in the following high-risk conditions:
(a)
Craniofacial anomalies (Pierre Robin sequence, achondroplasia)
(b)
Congenital CNS malformations (Arnold-Chiari, meningomyelocele, spina bifida)
(c)
Cerebral palsy
(d)
Neuromuscular diseases (Duchenne muscular dystrophy)
(e)
Obesity
(f)
Prader-Willi syndrome
(g)
Down syndrome
Intermediate risk conditions, in which the clinician should have a low threshold for ordering NPSG in the presence of signs/symptoms of SRBD, include the following:
(a)
Prematurity
(b)
African American race
(c)
Family history of SRBD
(d)
Sickle cell disease
However, there is insufficient evidence to support routine PSG in the following respiratory disorders unless there is a clinical suspicion for an accompanying sleep-related breathing disorder (O):
(a)
Chronic asthma
(b)
Cystic fibrosis
(c)
Bronchopulmonary dysplasia
(d)
Pulmonary hypertension
4.
PSG is indicated when the clinical assessment suggests the diagnosis of sleep-related hypoventilation due to neuromuscular or chest wall deformities (G).
5.
PSG is indicated when the clinical assessment suggests the diagnosis of congenital central alveolar hypoventilation syndrome (G).
Diagnostic Indications (Infants)
1.




PSG is indicated only in selected cases of primary sleep apnea of infancy (G). There is insufficient evidence to support the utility of routine PSG in establishing primary sleep apnea of infancy diagnosis and most infants are diagnosed by clinical history and direct observation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


