Ultrasound Techniques in Mediastinal, Lung, and Esophageal Disease
Carolyn E. Reed
The coupling of ultrasonography with the flexible esophagoscope and more recently the flexible bronchoscope has increased the ability to diagnose mediastinal disease and diagnose and stage lung and esophageal cancer via a minimally invasive approach.
Esophageal Ultrasonography in Lung Cancer
Esophageal endoscopic ultrasonography (EUS) can play an important role in the diagnosis of lung cancer, staging of the primary mass, mediastinal staging, and evaluation of certain sites of metastatic disease.
Equipment
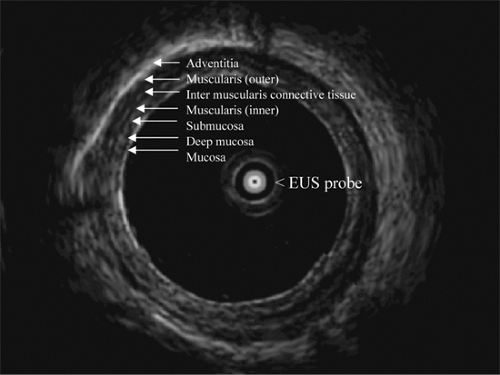
Initial evaluation and research studies in EUS utilized the radial echoendoscope to find lymph nodes, since it provided cross-sectional imaging similar to the computed tomography (CT) scan. However, radial echoendoscopes lacked the capability to provide tissue. Almost invariably, most endosonographers have now moved away from this practice and utilize curvilinear echoendoscopes that allow imaging and fine-needle aspiration (FNA) at the same time.
EUS–FNA Technique
Endoscopic ultrasound is performed under conscious sedation with the curvilinear array (CLA) echoendoscope. The examination starts by full evaluation of the left lobe of the liver and the left adrenal gland by imaging it from the fundus of the stomach. The descending aorta is identified with the CLA echoendoscope at about 35 cm from the incisor. A continuous and steady push of the CLA echoendoscope to about 45 cm from the incisor, while the aorta is maintained in view, leads to identification of the celiac axis bifurcation. A gentle clockwise maneuver will lead to the identification of a “seagull” shaped organ: the adrenal gland. In patients with metastasis to the adrenal, the gland loses its normal shape and takes the form of a mass. Then the echoendoscope is gradually withdrawn to evaluate the inferior pulmonary ligament nodal region (station 9), the periesophageal areas (station 8), the subcarinal space (station 7), the aortopulmonary window (station 5), and the upper and lower paratracheal area (stations 2 and 4, respectively). To identify the 4L station or even the aortopulmonary (AP) window (station 5), the endoscope is withdrawn from the gastroesophageal junction cephalad to just below the arch while keeping the descending aorta in view. At that particular location, and by torquing 90 degrees clockwise and tipping the up–down dial in the upward direction, the AP window is identified. It is, however, controversial whether the station identified is the AP window station or the 4L station. Once a lymph node is identified, EUS–FNA is performed with a curvilinear echoendoscope (Olympus UC-30P or UCT 140, Olympus, Melville, NY).
All EUS–FNAs are performed using 22-gauge adjustable length Echotip needles (Wilson-Cook Inc., Winston Salem, NC), or the Olympus Ezshot needle. Occasionally, when a core tissue is required, as in the diagnosis of lymphoma, core tissue is obtained utilizing 19-gauge Trucut needle.12 The procedure carries a very small risk of mediastinitis, bleeding, or stridor in patients with lung disease. Antibiotics are not routinely needed when lymph nodes are being sampled.
Staging the Primary Mass
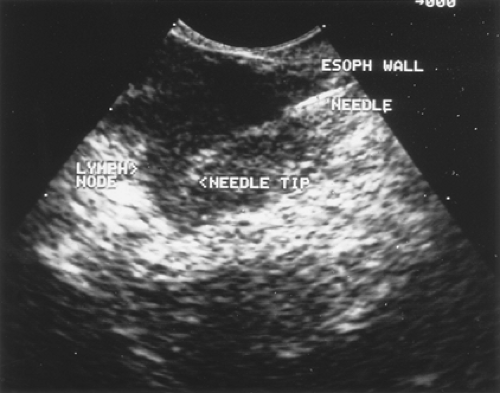
EUS, through the esophageal window, is able to assess the relationship of the tumor to the mediastinal vasculature in only a minority of patients owing to its limited penetration in the mediastinum. However, EUS can provide valuable information for vascular invasion when a tumor is seen (Fig. 14-1). One study evaluated 175 patients with lung cancer who underwent EUS.45 Ten patients were found by EUS to have stage T4 tumors; 7 were confirmed to be T4 by either surgical exploration (n = 2), CT demonstration of aortic invasion (n = 3), or documentation of malignant pleural effusion (n = 2). Three of the 10 (30%) patients reported as having stage T4 tumors by EUS had T2 disease at surgery and underwent curative resection. Of the remaining
165 patients without evidence of T4 disease at EUS, only one was found to have aortic invasion (T4) at surgery. EUS had a sensitivity of 87.5%, specificity of 98%, positive predictive value of 70%, and negative predictive value of 99% for detecting T4 disease.45
165 patients without evidence of T4 disease at EUS, only one was found to have aortic invasion (T4) at surgery. EUS had a sensitivity of 87.5%, specificity of 98%, positive predictive value of 70%, and negative predictive value of 99% for detecting T4 disease.45
Staging the Mediastinum
EUS–FNA is particularly useful for sampling inferior pulmonary ligament, esophageal, subcarinal, and aortopulmonary window lymph nodes (stations 9, 8, 7, and 5). Nodes anterolateral to the trachea are more difficult to sample reliably.
In a recent comprehensive meta-analysis, results of 18 studies were collated.32 EUS–FNA had a pooled sensitivity of 83% (95% CI, 78%–87%) and a specificity of 97% (95% CI, 96%–98%). For patients with enlarged lymph nodes seen on CT (n = 560), the pooled sensitivity was 90% (95% CI, 84%–94%); the specificity was 97% (95% CT, 95%–98%). For patients without enlarged lymph nodes on CT (n = 175), the pooled sensitivity of EUS–FNA was 58% (95% CI, 39%–75%), and the pooled specificity was 98% (95% CI, 96%–99%). This difference in diagnostic accuracy between studies enrolling patients with adenopathy on CT and those without adenopathy was statistically significant (p = 0.01).
Few studies have compared CT, PET, and EUS performance in staging the mediastinum. A study from Germany that included 33 patients showed that EUS–FNA was superior to CT and positron emission tomography (PET) combined.17 A larger study from the United States enrolled 104 patients with proven or suspected lung cancer who, by PET or CT, had an abnormal lymph node in the posterior mediastinum (station 5, 7, 8, or 9). These patients were then referred for EUS–FNA to sample posterior mediastinal lymph nodes after EUS and PET scanning.10 The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EUS–FNA were 92.5%, 100%, 100%, 94%, and 97%, respectively. EUS–FNA was more accurate and had a higher positive predictive value than the PET or CT scanning (p = 0.001) in confirming cancer in the posterior mediastinal lymph nodes.
Evaluating the Left Adrenal Gland
In addition to identifying nodal involvement, EUS–FNA can reliably sample the left lobe of the liver and the left adrenal gland (Fig. 14-2) for metastasis, resulting in a significant impact on patient management. EUS can identify the left adrenal gland in 97% of cases.7,9 In a recent multicenter study, EUS–FNA of the left adrenal was performed in 31 patients with thoracic or gastrointestinal (GI) malignancies.13 Tissue adequate for interpretation was obtained in all patients; the median number of needle passes was 4.5 (range 1–8). No immediate complications were encountered. EUS-guided FNA confirmed malignant left adrenal involvement in 42% (13 of 31) of the patients. Patients with malignant left adrenal masses were more likely to have known cancer at another site. Patients with benign masses were more likely to have preservation of the normal sonographic appearance of the adrenal gland (“seagull” configuration) compared with those with malignant masses. The accuracy of EUS imaging based on size (≥3 cm) alone was 81%. The authors concluded EUS-guided FNA of the left adrenal gland is a minimally invasive, safe, and highly accurate method that confirms or excludes malignant adrenal involvement in patients with thoracic or GI malignancies. The complication rate of the transgastric EUS-guided FNA approach appears to be much lower than that of the transcutaneous approach, since no organs are traversed except the wall of the stomach.
Restaging
EUS–FNA can sample mediastinal lymph nodes after chemotherapy and radiation to identify responders. Two studies, although with small numbers, confirm that the concept is feasible. The first study enrolled 19 patients.3 The positive predictive value, negative predictive value, sensitivity, specificity, and diagnostic accuracy of EUS–FNA for mediastinal restaging after induction chemotherapy were 100%, 67%, 75%,
100%, and 83%, respectively. Similar results were shown by Varadarajulu and colleagues.44 Fourteen patients were enrolled in this study. Restaging by EUS suggested disease response in 7 patients and residual disease in 6; tissue yield was unsatisfactory in 1 patient. Final diagnosis on the 7 patients in whom EUS suggested N0 disease was established at surgery: EUS was true negative in 6 and false negative in 1. The diagnostic accuracy of EUS–FNA for predicting mediastinal response to preoperative therapy was 86%.44 EUS–FNA appears to be an accurate, safe, and minimally invasive diagnostic technique for the restaging of mediastinal lymph nodes in patients with non-small-cell lung cancer.
100%, and 83%, respectively. Similar results were shown by Varadarajulu and colleagues.44 Fourteen patients were enrolled in this study. Restaging by EUS suggested disease response in 7 patients and residual disease in 6; tissue yield was unsatisfactory in 1 patient. Final diagnosis on the 7 patients in whom EUS suggested N0 disease was established at surgery: EUS was true negative in 6 and false negative in 1. The diagnostic accuracy of EUS–FNA for predicting mediastinal response to preoperative therapy was 86%.44 EUS–FNA appears to be an accurate, safe, and minimally invasive diagnostic technique for the restaging of mediastinal lymph nodes in patients with non-small-cell lung cancer.
Endobronchial Ultrasound (EBUS) in Lung Cancer
The recent development and use of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS–TBNA) adds another minimally invasive tool for the diagnosis and staging of intrathoracic malignancy. EBUS–TBNA reaches multiple lymph node stations, including high paratracheal levels (levels 2 and 3), a lower paratracheal level (level 4), a subcarinal (level 7), and even hilar nodes (levels 10 and 11). When combined with EUS, the combination can provide nearly complete staging of the mediastinum, with a negative predictive value of 100% reported by Wallace and associates.51
EBUS–TBNA Technique
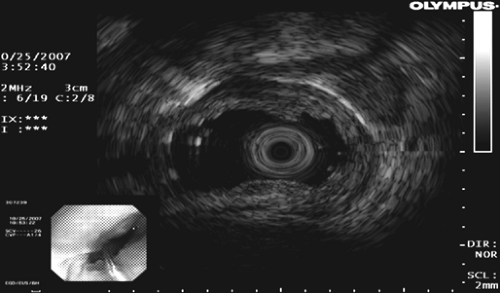
Bronchoscopy is performed with a flexible videobronchoscope equipped with a 7.5-MHz linear ultrasound transducer probe, as described by Vilmann and colleagues47 and illustrated by Herth and associates.23 All accessible lymph node stations are examined. Based on prior imaging studies (CT, PET, CT/PET), lymph node biopsy is carried out, starting with the nodal level that would yield the highest-stage disease first. This avoids serial contamination from a positive node that would not upstage a patient’s disease. A 22-gauge needle designed for the videoendoscope is placed through the biopsy channel to puncture the lymph node under real-time ultrasonic guidance (Fig. 14-3). A Doppler examination is carried out just prior to the biopsy to prevent unintended puncture of vessels. Multiple passes (3–5) are made. TBNA thin-smear samples are prepared on site by a cytology technician. Many centers use a rapid on-site pathologist to evaluate adequacy of the specimen (presence of lymphocytes) and thereby improve the yield.
EBUS–TBNA is performed in the outpatient setting under local anesthesia. Serious adverse events have not been reported.
Results of EBUS–TBNA
TBNA alone is limited in that it is a blind technique and yield is related to the size and location of the lesion. In general, it has been underutilized. Reported sensitivity averages 76% (range 14%–100%) and negative predictive value (NPV) averages 71% (range 36%–100%).42
Yasufuku and associates54 demonstrated the usefulness and safety of real-time EBUS-guided TBNA in the evaluation of mediastinal and hilar adenopathy and reported their results in 70 patients in 2004. The accuracy of distinguishing benign from malignant lymph nodes was 97.1%. Herth and colleagues20 entered 242 consecutive patients into a prospective study evaluating the feasibility of EBUS for TBNA guidance and demonstrated successful lymph node assessment in 86% of patients. The diagnostic yield was 71%. In a randomized trial of 200 patients, Herth et al.19 showed that EBUS guidance significantly increased the yield of TBNA in all lymph node stations except in the subcarinal region.
Recent series reporting the results of EBUS–FNA for the mediastinal lymph node staging of lung cancer are detailed in Table 14-1. With the exception of the study by Wallace et al.,51 the sensitivities and NPVs have been high. Of note is a study by Herth et al.22 evaluating the accuracy of EBUS–TBNA in 100 non-small-cell lung cancer patients with CT showing no enlarged lymph nodes (all nodes ≤1 cm in diameter). Malignancy was detected in 19 patients and missed in two. The sensitivity and NPV of EBUS–TBNA for detecting malignancy in a radiologically normal mediastinum was 92.3% and 96.3%, respectively. In a subset of patients with both a CT- and PET-negative mediastinum reported by Wallace and associates,51 the NPV was 89%.
Table 14-1 Results of EBUS-TBNA in Mediastinal Lymph Node Staging for Lung Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EBUS–TBNA Combined with EUS–FNA in Lung Cancer
EBUS–TBNA and EUS–FNA are clearly complementary staging modalities whereby EBUS has access to the paratracheal, subcarinal, and hilar regions and EUS to the lower mediastinum and aortopulmonary window. The accuracy of the combination of EBUS–TBNA and EUS–FNA has been reported to be 100%.47 In a study with a crossover design of patients with enlarged lymph nodes in one of eight lymph node stations, EBUS–TBNA was successful in 85% and EUS–FNA in 78%.24 Combining both approaches produced successful biopsies in 97% and diagnoses in 94%. Wallace et al.51 confirmed that the combination of EUS–FNA and EBUS–TBNA achieves a near complete “medical” mediastinal staging.
The staging of the mediastinum is critical for optimal management of non-small-cell lung cancer. The combined use of EBUS–TBNA and EUS–FNA offers the potential of more comprehensive access to mediastinal and even hilar lymph nodes than is usual by standard mediastinoscopy. These minimally invasive approaches avoid general anesthesia, can be performed in the outpatient setting, and reduce risk. The same lymph node level can be repeatedly biopsied, which may be important if downstaging is essential to surgical intervention or assessment of therapeutic effect is desired. Despite a negative evaluation of CT-enlarged or PET-positive mediastinal lymph nodes by EBUS–TBNA and/or EUS–FNA, there will be instances when more invasive staging is warranted by tumor characteristics, surgeon judgment, or patient risk profile.
Role of EUS–FNA in Differentiating Mediastinal Cysts from Solid Masses
EUS-guided FNA is an ideal minimally invasive tool for evaluating posterior mediastinal masses. The primary role for EUS is to differentiate cystic lesions (bronchogenic or duplication cysts) from solid mediastinal masses.11 Bronchogenic cysts usually reveal one of two echogenic patterns: anechoic and simple (the majority) or anechoic admixed with solid debris. Clear liquid can often be aspirated from simple anechoic cysts, but aspiration of simple cysts is not advocated since they have a classic appearance by EUS and are accurately classified by CT. The approach to heterogeneous cysts is not as straightforward. These cysts are usually filled with thick echogenic, tenacious debris; occasionally hyperechoic reflectors can be seen. Proper aspiration technique usually results in a frothy, brownish fluid, but the high viscosity can sharply limit the yield to just a few drops for interpretation. It is important to realize that these types of cysts are often incorrectly interpreted as solid masses by cross-sectional imaging (CT or MRI). The main indication to aspirate such lesions is to rule out a cystic metastasis. Prophylactic antibiotics should be given, as there have been case reports of infection of mediastinal cysts without antibiotic coverage.4,15,16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree