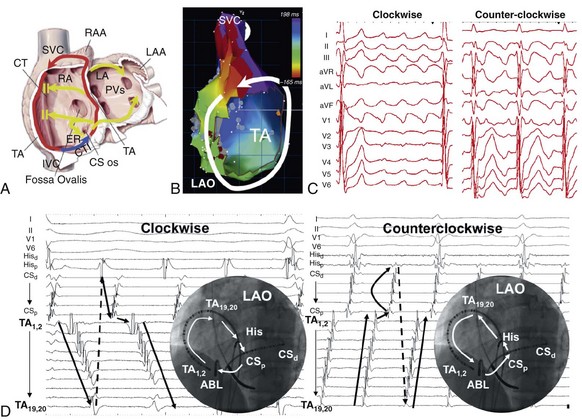
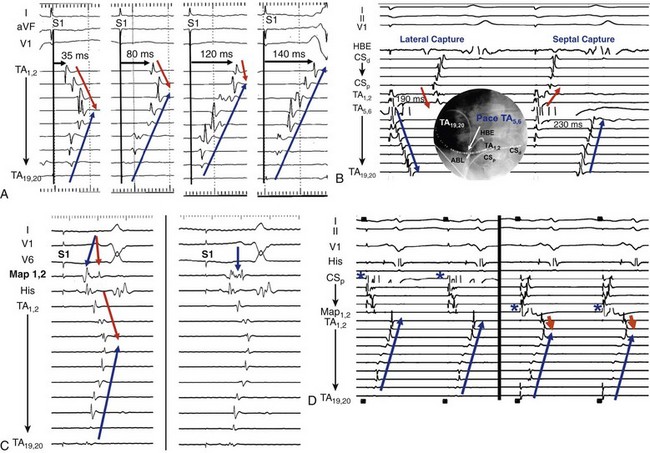
74 Atrial Flutter as a Reentrant Arrhythmia: Lessons from Animal Studies Classification of Atrial Flutter Cavotricuspid Isthmus–Dependent Flutter Noncavotricuspid Isthmus–Dependent Flutter (Atypical Flutter) Right Atrial Atypical Flutter (No Prior Surgery) Left Atrial Atypical Flutter (No Prior Surgery) Postsurgical or Scar-Mediated Flutter Atrial flutter (AFL) is one of the most common cardiac arrhythmias in humans, affecting approximately 190,000 people in the United States in 2005; its prevalence is expected to increase to 440,000 by 2050 because of the increasingly older population.1 AFL usually coexists with atrial fibrillation (AF) and is generally initiated through a transitional phase of AF.2 AFL most often occurs in the context of structural heart disease (e.g., valvular heart disease, ischemic heart disease, cardiomyopathy), but can also manifest during an acute disease process such as respiratory infection or myocardial infarction. Significant advances have been made in the understanding of the mechanism of AFL, its heterogeneous nature, and its treatment, which will be reviewed in this chapter. AFL is defined here as an arrhythmia with a macroreentrant circuit (>2 cm) distinct from focal atrial tachycardias (or small circuit reentry), with subsequent centrifugal spread.3 Reentry as the underlying mechanism of AFL was suggested by observations that although AFL induction and sustainability was difficult in the normal canine atria, it could be reliably induced and sustained in the presence of a linear atrial lesion between the venae cavae. A line of block between the superior and inferior vena cava provided an obstacle around which the wavefront could circulate and was critical in preventing short-circuiting of the AFL circuit.2 Additional work showed that this line of block might not be limited to the intercaval region and need not be fixed (anatomic), but instead could be functional. A right atrial free wall lesion, such as an atriotomy scar, might be a central obstacle around which a wavefront circulates sometimes termed incisional reentry. However, functional extension of a fixed line of block (seen in an animal model) can result in a circuit similar to typical atrial flutter where the extended line of block forms a posterior barrier. In the absence of the functional extension of the anatomic line of block, reentry was noted to occur around the lesion alone.2 These findings explain the mechanism for the coexistence of classical cavotricuspid (CTI)-dependent AFL and incisional reentrant AFL, and the conversion from one arrhythmia to the other after development of CTI block with catheter ablation. Animal and human studies of induced and spontaneous AFL have shown that AFL is usually preceded by a transitional period of AF.2 It has been suggested that the critical functional line of block between the venae cavae develops during transitional AF, thereby establishing the necessary boundaries for reentry required for AFL maintenance. The absence of the functional line of block results in either in persistence (no conversion to AFL) or termination of AF. Conversely, shortening of the line of block results in conversion of AFL to AF. The coexistence of both arrhythmias is evidenced by the fact that nearly 75% of patients with AFL will have had documented AF at the time of presentation.2 Furthermore, the incidence of AF after an AFL ablation is time dependent, with one study finding an 82% incidence of new-onset, drug-refractory AF during a 39-month follow-up.4 Indeed, AFL ablation alone does not modify the natural course of arrhythmia progression to AF, and data suggest that a high percentage of patients will ultimately develop AF after ablation of AFL. Furthermore, antiarrhythmic drugs causing atrial conduction slowing (class Ia, class Ic, amiodarone) can convert AF to AFL in part related to the creation of a posterior or intercaval functional line.2 Although AF and AFL frequently coexist, in the clinical presentation one arrhythmia frequently predominates over another. The predominance of AFL presentation over AF in some patients might be explained by more advanced right atrial remodeling with slowed conduction and regional conduction block, particularly occurring in the posterior right atrium (RA). This distribution of remodeling can facilitate the stabilization of AFL and facilitate persistence of this as the dominant clinical arrhythmia.5 Furthermore, when studied remote from episodes of arrhythmia, patients with AFL demonstrate significant and diffuse atrial abnormalities characterized by atrial structural changes, conduction abnormalities, and sinus node dysfunction. These changes might explain the predisposition to the development of AFL and the subsequent propensity for AF.6 The nomenclature around atrial macroreentrant arrhythmia classification continues to be confusing. In a statement from a joint expert working group of the European Society of Cardiology and then the North American Society of Pacing and Electrophysiology in 2001, it was suggested that all macroreentrant arrhythmias be classified as “macroreentrant atrial tachycardia” and that the term AFL be used as a descriptive moniker referring to a continuously undulating pattern on the electrocardiogram (ECG), without isoelectric baseline in at least one lead. In practice, however, the terms continue to be used interchangeably, with the use of the historical term atrial flutter remaining predominant. A suggested AFL classification is shown in Box 74-1, with broad division into those that are dependent on the CTI-dependent and those that are not (atypical or non–CTI dependent).7 These arrhythmias are fundamentally underpinned by the classic notion of reentry around a central obstacle (either a fixed anatomic structure or a line of functional block) and the presence of slow conduction to create an excitable gap. Atypical AFL comprises a heterogeneous group of arrhythmias that are not isthmus dependent, could arise from either atria, are composed of circuits that are highly variable and involve a range of anatomic boundaries. Broadly, atypical AFL can occur in the context of previous atrial surgery (congenital, valvular heart disease, MAZE procedures), after catheter ablation for AF, cardiac transplantation, or in the absence of previous atrial surgery.8 Mapping of atypical flutter occurring in the absence of prior surgery can nevertheless demonstrate regions of low-voltage amplitude and scarring critical to the arrhythmia mechanism. This can occur in the context of structural heart disease, such as heart failure or valvular regurgitation, in which the mechanism of scarring can be attributed to chronic atrial stretch. Occasionally, such scarring is seen in the absence of structural heart disease where the mechanism is unknown. The circuits of counterclockwise and clockwise atrial flutter can be characterized as right atrial with a broad activation wavefront rotating between the tricuspid annulus (TA) as the anterior barrier and the crista terminalis-eustachian ridge/inferior vena cava (IVC) as the posterior barrier in either a counterclockwise or a clockwise direction. The CTI provides the narrowest segment of the circuit (Figure 74-1, A, B).9,10 Although these are the critical barriers, significant variations in the leading edge or active region of the reentrant loop have been described. In an entrainment study, Santucci et al.11 demonstrated that only approximately one third of typical AFL cases had an active circuit adjacent to the TA. In others, the circuit took an oblique course between the anterior and posterior barriers, with the upper circuit off the annulus and posterior to the right atrial appendage (RAA) base. Of these, some coursed anterior to the superior vena cava (SVC), others behind the SVC (across the crista terminalis). In others, bifurcation of the upper circuit was seen around the RAA, around the SVC, or around both.11 Figure 74-1 CTI-dependent flutter. A, Schematic depicting the circuit of typical counterclockwise AFL. The RA is seen anteroposteriorly with the anterior surface removed. Arrows represent the atrial activation sequence during AFL. Red arrows identify components that are part of the circuit, and yellow arrows mark components activated passively outside the circuit. Areas of conduction block are marked with double lines (e.g., crista terminalis, eustachian ridge), providing the adequate path length for the flutter reentry circuit and preventing its short-circuiting. B, Three-dimensional electroanatomic activation map of the RA during counterclockwise AFL shows early to late activation around the tricuspid annulus using a color spectrum from red (marking early) to purple (marking late) as an arbitrary reference. C, Characteristic P wave morphology of clockwise and counterclockwise AFL, which are best observed during periods of AV block or during ventricular pacing to unencumber the P waves. D, Intracardiac electrograms and fluoroscopic left anterior oblique view of multipolar catheters (insets) documenting the circuit of clockwise and counterclockwise AFL. Arrows depict direction of atrial activation during AFL. AFL, Atrial flutter; CS, coronary sinus; CT, crista terminalis; CTI, cavotricuspid isthmus; d, distal; His, His bundle electrogram; IVC, inferior vena cava; LA, left atrial; p, proximal; PVs, pulmonary veins; RA, right atrium; RAA, right atrial appendage; SVC, superior vena cava. (C, From Medi C, Kalman JM: Prediction of the atrial flutter circuit location from the surface electrocardiogram. Europace 10:786–796, 2008.) Counterclockwise typical AFL is the most common form of macroreentrant AFL. The classical inferior lead flutter waves (sawtooth pattern) demonstrate an initial gradual downsloping segment followed by a deeply inverted component with a terminal positive component (of variable amplitude; see Figure 74-1, C). Although the flutter wave typically appears inverted in inferior leads, variations in the amplitude of both the negative and positive components can create atypical patterns. In the precordial leads, V1 classically demonstrates an initial isoelectric component followed by an upright component. With progression across the precordial leads, the initial component becomes inverted, and second component becomes isoelectric such that V5 and V6 demonstrate an inverted flutter wave.12 Lead I is low amplitude–isoelectric, and aVL is usually upright (see Figure 74-1, C). Unusual flutter wave morphologies can be seen with counterclockwise AFL and a left AFL might mimic the counterclockwise AFL appearance. Clockwise AFL is seen in approximately 10% of cases, and its boundaries are identical to counterclockwise AFL, but with reverse direction of wavefront rotation. The surface ECG appearance tends to be more variable, but with some characteristic features. Flutter waves in the inferior leads are usually broadly positive, and the upright component is preceded by an inverted component of variable amplitude. There is characteristic notching on the upright component, which has a bifid appearance. V1 is characterized by a broad, negative, and usually notched deflection with transition to an upright deflection in V6. Lead I is usually upright and aVL is low amplitude negative, overall giving an appearance that is the reverse of counterclockwise AFL (see Figure 74-1, C). Although the ECG appearance of the flutter waves may be highly characteristic, it is always important to confirm the arrhythmia mechanism with activation and entrainment mapping before performing ablation. The RA activation sequence during counterclockwise and clockwise AFL can be delineated with multipolar catheters or the use of high-density three-dimensional (3D) electroanatomic mapping systems. Typically, multipolar catheters are placed in the coronary sinus (CS), around the TA and in the His bundle position (see Figure 74-1, D). In patients with paroxysmal AFL who are in sinus rhythm, rapid atrial pacing can be used to initiate the arrhythmia, although AF might also ensue. Onset is characteristically with unidirectional block within the CTI such that pacing from the CS ostium most usually will induce counterclockwise AFL, whereas low lateral RA pacing will most usually induce clockwise AFL. During transient entrainment of a tachycardia with a pacing cycle length 10 to 20 ms shorter than the flutter cycle length, the wavefront from each pacing impulse orthodromic and antidromic fashion.13 The antidromic wavefront will collide with the preceding orthodromic wavefront as it exits the critical isthmus. Entrainment will result in acceleration of the atrium to the pacing cycle length, with each antidromic wavefront colliding with the orthodromic wavefront of the preceding pacing stimulus (constant fusion). When pacing stops, the orthodromic wavefront of the last pacing stimulus has a cycle length equal to the cycle length of entrainment, but as it does not encounter a further pacing-induced antidromic wavefront, it is not fused (the last entrained but not fused beat). When pacing rate is further increased, the collision site between orthodromic and antidromic wavefronts is shifted farther from the pacing site so that more of the ECG complex appears paced (progressive ECG fusion) and more electrogram sites are antidromically captured (progressive electrogram fusion). When entrainment is performed in a narrow isthmus, the extent of antidromic capture is limited within the isthmus, and fusion will not be evident on the surface ECG (concealed entrainment; Figure 74-2). When outside a protected isthmus antidromic atrial capture or penetration is more extensive and therefore manifest on the surface ECG (manifest fusion). Figure 74-2 Examples of entrainment from a patient with left atrial flutter. A, Pacing the lateral cavotricuspid isthmus (CTI) result at 230 ms results in acceleration of the tachycardia and paced activation sequence on the tricuspid annulus (TA) catheter that is different from the tachycardia (manifest fusion). Note that the apparent counterclockwise activation pattern on the TA catheter (19,20 toward 1,2) suggests a counterclockwise mechanism. Simple entrainment from within the CTI demonstrates extensive antidromic penetration up the lateral wall of the right atrium, although pacing is only 15 ms less than the TCL, indicating that the pacing site is distant from the circuit. This finding is confirmed by the postpacing interval (PPI; 95 ms longer than TCL). B, Pacing from the ablation catheter positioned in the mid coronary sinus shows endocardial concealment (endocardial activation pattern visually unchanged) and a PPI equal to the TCL, indicating a site within the circuit. Simple entrainment rapidly excluded the right atrium as the site of the circuit. High-density, 3D electroanatomic mapping (contact or noncontact mapping) is able to delineate the right atrial circuit during typical AFL with the use of activation maps represented as a continuous progression of colors (see Figure 74-1, B). A key point with activation mapping using electroanatomic mapping systems in macroreentrant circuits is that early activation is not applicable to any specific point in the circuit, because activation is continuous and the origin of activation is always arbitrary with respect to the particular reference chosen for illustration. Therefore, color coding will be represented in red or white at the site with earliest activation with respect to the arbitrarily defined zero point and will progress through a spectrum of colors to purple representing latest activation, again with respect to the arbitrarily chosen point. Activation timing should span the entire cycle length of a macroreentrant circuit. Where earliest meets latest activation in a continuous circuit is the point where “head meets tail,” which is again an arbitrary anatomic point determined by choice of the zero point. Using 3D mapping, the activation wavefront in counterclockwise AFL will most typically appear as a broad wavefront that exits the medial CTI, ascends the septum, passes anterior to the SVC, and descends the anterolateral RA to enter the CTI laterally (see Figure 74-1, B). Passive wavefronts will activate the left atrium and the posterior RA, where conduction delay or block at the crista terminalis will prevent a short-circuiting of the TA (see Figure 74-1).9,10 As described earlier, entrainment mapping and noncontact mapping have both been used to define the many variants on the location of the active circuit of typical AFL. 3D mapping can also characterize tissue voltage of the CTI, which could be useful in planning the path of the ablation line because lower voltage can predict an easier ablation path.14 Lower loop reentry is also a CTI-dependent flutter but has a shorter circuit confined to the lower RA with rotation around the IVC in a clockwise or counterclockwise fashion. It is created by the presence of a posterior breakthrough at the crista terminalis and often coexists with typical AFL around the TA.15 The circuit exits the medial CTI, with activation proceeding across the posterior RA. Transverse conduction across the crista terminalis occurs usually at a site of critical slow conduction. Clockwise variants of lower loop reentry have also been described. The true prevalence of lower loop reentry is uncertain as the ECG morphology can be indistinguishable from typical flutter and termination occurs with CTI ablation. It is therefore possibly more common than diagnosed. Subtle differences in flutter wave morphology from typical flutter are determined by the level of breakthrough at the crista terminalis. Low lateral RA breakthrough will result in attenuation of the late positive deflection of the flutter wave compared with that of counterclockwise typical AFL. Intraisthmus reentry is a circuit that revolves around the medial CTI and coronary sinus ostium; the lateral isthmus is not part of the circuit. Concealed entrainment is demonstrable in the medial CTI or the coronary sinus ostium, but not the lateral CTI, and other sites around the TA are out of the circuit.16 Double wave reentry is induced when a critically timed, atrial extra stimulus is introduced into an AFL circuit with a wide excitable gap, resulting in a second excitation wavefront such that two wavefronts coexist within the circuit simultaneously.17 The phenomenon manifests as acceleration of the flutter cycle length with identical surface and intracardiac electrogram morphology and simultaneous activation of the superior and inferior TA. Generally, this rhythm lasts only a few beats and can trigger AF. Principles of pharmacologic treatment are to control the ventricular rate during AFL with the use of atrioventricular (AV) node blocking agents (β-blockers, cardiac-selective calcium channel blockers) or maintenance of sinus rhythm with class I or III antiarrhythmic drugs. Class 1c agents can stabilize the flutter by slowing rate, increasing excitable gap, and can result in 1 : 1 AV conduction. Because of the presence of postreversion atrial stunning, whether pharmacologic or electrical, it is mandatory to follow anticoagulation guidelines as for reversion of AF. Because of the high late incidence of AF after successful flutter ablation coupled with the documented late risk of stroke,18 it is common to make decisions regarding ongoing anticoagulation (with coumadin or an equivalent) based on the CHADS2 score. In the absence of supporting data, an alternate approach is to continue to monitor closely for development of late AF particularly in those patients with stroke risk factors. Pharmacologic treatment results in long-term maintenance of sinus rhythm in 36% to 73% of patients with AFL; moreover, complete arrhythmia suppression can be difficult.19 Evidence from randomized studies has demonstrated the superiority of catheter ablation over antiarrhythmic drugs in the treatment of AFL, with higher rates of maintenance of sinus rhythm, improvement in quality of life, and reduction in the recurrence of future AF.20,21 Thus, catheter ablation is a class I recommendation for AFL if it is recurrent, is poorly tolerated, or reemerges after using a class I antiarrhythmic or amiodarone.19 Catheter ablation can be performed in either the septal or mid aspects of the CTI and will be determined by stability of catheter access and anatomic variation (see Figure 74-1, D). It is important to be aware that inadvertent AV block has rarely been reported during ablation at the septal aspect of the CTI because of the proximity to posterior extensions of the AV node. The lateral isthmus is generally longer and is not usually the primary ablation target. Ablation can be performed during AFL or proximal CS pacing (in sinus rhythm). The latter allows identification of a change in activation sequence on the tricuspid annular catheter, signifying slowing of CTI conduction or block (Figure 74-3, A). Figure 74-3 Methods of assessing block across the cavotricuspid isthmus (CTI). A, Coronary sinus (CS) pacing (representing pacing septal to the CTI) is depicted before CTI block (first three panels). A clockwise wavefront traveling across the CTI from the distal to proximal tricuspid annulus (TA) collides with the counterclockwise wavefront ascending up the interatrial septum and down from the proximal to the distal TA. During ablation, there is a progressive increase in transisthmus conduction (first three panels) until CTI block is achieved (last panel). Note that in the last panel there is a change in activation sequence on the TA catheter where the distal TA located lateral to the CTI is activated last and there is reversal of electrogram polarity. B, Inset
Typical and Atypical Atrial Flutter
Mapping and Ablation
Atrial Flutter as a Reentrant Arrhythmia: Lessons from Animal Studies
Interdependence of Atrial Fibrillation and Atrial Flutter
Classification of Atrial Flutter
Cavotricuspid Isthmus–Dependent Flutter
Counterclockwise and Clockwise Atrial Flutter (Typical Flutter)
Mapping
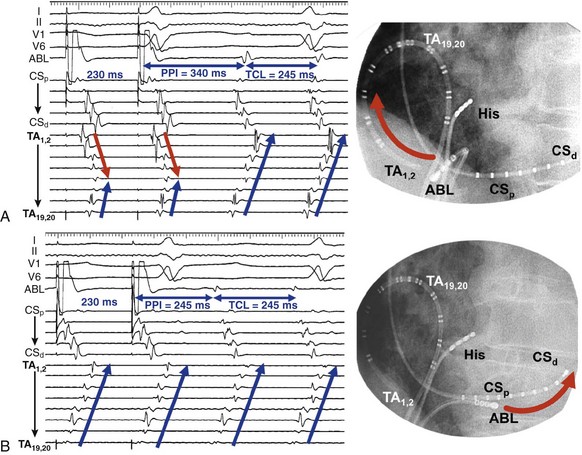
Entrainment
Electroanatomic Mapping
Lower Loop Reentry
Intraisthmus Reentry
Double Wave Reentry
Treatment
Pharmacologic
Catheter ablation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


