CHAPTER 121 Truncus Arteriosus and Aortopulmonary Window
TRUNCUS ARTERIOSUS
Truncus arteriosus is a relatively rare congenital heart defect with a single vascular trunk arising from the heart giving origin to the true pulmonary arteries, aorta, coronary arteries, and brachiocephalic vessels. The lesion accounts for approximately 0.4% to 4.0% of all congenital heart lesions.1–3 Truncus arteriosus was first described by Wilson4 in 1798. In 1942, Lev and Saphir5 defined the anatomy currently associated with truncus arteriosus.
Embryology
Pluripotent neural crest cells play an important role in the development of the conotruncus and aortic arch. Selective ablation of neural crest cells in chick embryos before migration results in a number of congenital heart defects.6 These anomalies include truncus arteriosus and interrupted aortic arch, explaining their coexistence in approximately 10% to 20% of patients with truncus arteriosus.7,8
Animal models provide further insight into the genetic basis for truncus arteriosus. The mouse mutant Splotch, which has a mutation in the homeobox gene Pax3, has a phenotype with truncus arteriosus and aortic arch abnormalities.9 In humans, monoallelic microdeletion of chromosome 22q11 is associated with multiple defects of neural crest origin, including typical facies, cleft palate, thyroid and parathyroid gland aplasia, and conotruncal and aortic arch abnormalities. The resulting phenotypic syndromes include DiGeorge, velocardiofacial, and Shprintzen syndromes, which are associated with truncus arteriosus. One candidate gene identified in the area of the microdeletion is HIRA. HIRA interacts with Pax3, and thus may be integral to Pax3 regulation of neural crest cells.10 Environmental risk factors for the development of truncus arteriosus include maternal diabetes and exposure to retinoic acid.
Anatomy
In truncus arteriosus, there is generally situs solitus and D-looping of the ventricles. A single great vessel arises from the base of the heart, giving origin to the pulmonary, systemic, and coronary arteries. Classifications of truncus arteriosus were proposed by Collett and Edwards11 in 1949 and by Van Praagh and Van Praagh12 in 1965 (Fig. 121–1). The system described by Collett and Edwards (Table 121–1) is based on the site of origin of the pulmonary arteries, whereas the Van Praagh classification (Table 121–2) is based on the degree of septation of the trunk and the presence or absence of a VSD. The Van Praagh scheme also requires that at least one of the pulmonary arteries arise from the common trunk. This stipulation appropriately relegates Collett-Edwards type IV, or pseudotruncus, to the spectrum of pulmonary atresia with aortopulmonary collaterals. The Van Praagh classification also provides for the inclusion of the relatively common association of hypoplastic or interrupted aortic arch. The term hemitruncus is frequently encountered in the literature to describe the anomalous origin of the right pulmonary artery from the ascending aorta with a normal origin of the left pulmonary artery from the main pulmonary artery, usually in the absence of a VSD. This lesion is distinct from Van Praagh type B3 and should not be considered a form of truncus arteriosus.
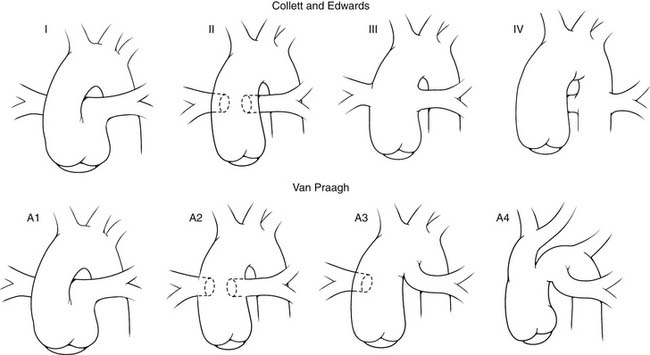
Figure 121–1 Comparison of the Collett and Edwards and the Van Praagh and Van Praagh classifications of truncus arteriosus.
Table 121–1 Collett and Edwards Classification
| Type I | Branch pulmonary arteries arise from a segment of the main pulmonary artery off the common trunk. |
| Type II | Branch pulmonary arteries arise in close proximity from the posterior aspect of the common trunk. |
| Type III | Branch pulmonary arteries arise from separate, widely spaced origins. |
| Type IV | Absent “true” branch pulmonary arteries with aortopulmonary collaterals |
Table 121–2 Van Praagh and Van Praagh Classification
| Type A | Ventricular septal defect present |
| Type B | Ventricular septal defect absent |
| 1 | Partial development of the aorticopulmonary septum |
| 2 | Absence of the aorticopulmonary septum |
| 3 | Absence of one of the branch pulmonary arteries |
| 4 | Coarctation, hypoplasia, or interruption of the aortic arch with a patent ductus arteriosus |
A VSD is nearly always present. It results from the deficiency of the infundibular septum and is generally nonrestrictive. The VSD is cradled by the two limbs of the septal band and bounded superiorly by the truncal valve (Fig. 121–2). The posterior (inferior) limb of the septal band usually inserts into the parietal band, resulting in discontinuity of the tricuspid and truncal valves, maintaining a muscular rim between the conduction system and the VSD. When there is failure of insertion, the VSD extends into the membranous septum, with the conduction system running along the posterior-inferior rim of the defect.
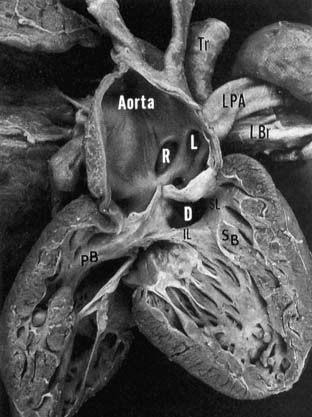
Figure 121–2 Right ventricular view of the pathologic anatomy of truncus arteriosus. The truncal origins of the aorta and left (L) and right (R) pulmonary arteries are apparent. The ventricular septal defect (D) is cradled between the inferior (IL) and superior (SL) limbs of the septal band (SB). Insertion of the IL into the parietal band (PB) provides muscular discontinuity between the tricuspid and truncal valves. LBr, left bronchus; LPA, left pulmonary artery; Tr, trachea www.lww.com.
(Reprinted with permission from Mair DD, Edwards WD, Julsrud PR, et al. Truncus arteriosus. In: Allen HD, Gutgesell HP, Clark EB, Driscoll DJ, editors. Moss and Adams’ heart disease in infants, children and adolescents. Philadelphia: Lippincott, Williams & Wilkins; 2001, p. 505)
The truncal valve may be tricuspid (69%), quadricuspid (22%), bicuspid (9%), or, rarely, unicuspid or pentacuspid.13 The valve is usually in continuity with the mitral valve and infrequently with the tricuspid valve. The truncus equally overrides both ventricles in 68% to 83% of cases, is deviated over the right ventricle in 11% to 29%, and is deviated to the left in 4% to 6%.6,14 The valve may be stenotic or regurgitant, which can complicate management of the patient with truncus arteriosus. A moderate or greater degree of truncal insufficiency is present in 20% to 26% of patients.15,16 Mild stenosis is generally detected on preoperative evaluation because of the increased flow across the truncal valve. Significant stenosis is present in only 4% to 7%.15,16 Gradients of greater than 30 mm Hg are concerning for residual stenosis after complete repair.17
The branch pulmonary arteries generally originate from the left posterolateral aspect of the truncus, just distal to the truncal valve. They are usually of good size without ostial or branch stenosis. Collett and Edwards type I is most frequently encountered, occurring in 48% to 68% of cases, followed by type II (29% to 48%) and type III (6% to 10%).6,14 In practice, most cases seem to fall into a category of “type 1½,” with the branch pulmonary arteries arising not from a main pulmonary artery but in proximity from the posterior aspect of the truncus. Origin of one pulmonary artery from a systemic artery other than the truncus (Van Praagh type A3/B3) is relatively rare, with an incidence of 2% to 5%.15,18
Associated Anomalies
An interrupted aortic arch, most commonly type B, is present in association with truncus arteriosus (Van Praagh type A4/B4) in approximately 10% to 20% of patients.7,8 The arch is rightward, generally with mirror image branching, in 21% to 36%.7,19 Aberrant origins of the brachiocephalic vessels are reported, most commonly an aberrant right subclavian artery in 4% to 10%.14,19
Coronary variations are common in truncus arteriosus and are of potential importance in the surgical repair of the lesion. The left anterior descending artery is often small, with prominent conal branches from the right coronary artery supplying the right ventricular infundibulum.20 The left anterior descending artery can originate from the right coronary artery, which has clear surgical ramifications for the right ventricular infundibulotomy. There is left coronary dominance in 27%, which is approximately three times the incidence found in the general population.21 Coronary ostial abnormalities are ofparticular surgical significance and occur in 37% to 49% of cases.21 The usual arrangement, regardless of the number of cusps, is for the left coronary artery to arise from the left posterolateral cusp and the right to originate from the right anterolateral cusp. Coronaries may arise from a single orifice or from two ostia in a single cusp.7 There may be ostial stenosis, often described as a slit-like orifice, or obstruction from abnormal valve tissue. The left coronary artery is frequently noted to have a high origin, not uncommonly near the takeoff of the pulmonary arteries. Rarely, the left coronary artery can originate from the main pulmonary trunk or a branch pulmonary artery.14
Other cardiac anomalies are common, and a patent foramen ovale (PFO) is usually present. A true atrial septal defect is found in 9% to 20%, a persistent left superior vena cava in 4% to 9%, and mild tricuspid valve stenosis in 6%.14,19 Mitral valve abnormalities are reported in 5% to 10% of patients. Tricuspid atresia, complete atrioventricular septal defect, anomalies of pulmonary venous return, mitral atresia, ventricular inversion, and heterotaxy syndrome have all been reported in association with truncus arteriosus.
Extracardiac anomalies are reported in approximately 28% of patients with truncus arteriosus.16 Described abnormalities include skeletal, genitourinary, and gastrointestinal deformities. As mentioned earlier, monoallelic microdeletion of chromosome 22q11 is common, and DiGeorge syndrome is diagnosed in at least 11%.16
Diagnosis
Clinical Features
The diagnosis of truncus arteriosus is generally made in early infancy, often during the neonatal period. The lesion may also be recognized antenatally on fetal echocardiography. The degree of cyanosis or congestive heart failure depends on the PVR and the resultant volume of pulmonary blood flow. The clinical manifestations may be exacerbated by associated lesions, such as truncal valve insufficiency or interrupted aortic arch or may be ameliorated by pulmonary artery stenosis.
Diagnostic Studies
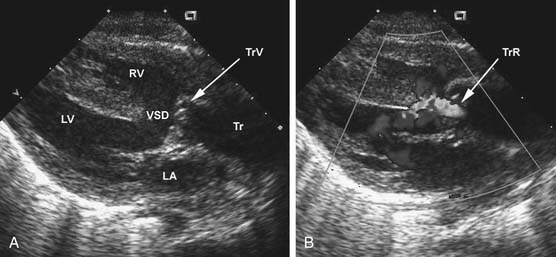
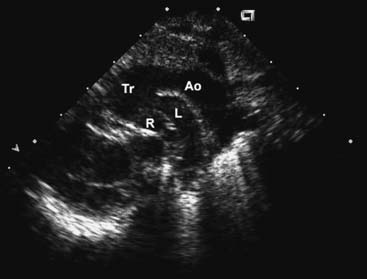
The chest radiograph generally shows moderate cardiomegaly with increased pulmonary vascular markings. The arch is rightward in approximately a third of patients, and the thymus gland may be absent in those with 22q11 microdeletion. The combination of a right arch and increased pulmonary vascular markings is strongly suggestive of truncus arteriosus. The two-dimensional and Doppler echocardiography examinations are the diagnostic modalities of choice. The echocardiogram can define the anatomy of truncus arteriosus at birth or in utero. A parasternal long-axis view will demonstrate the large truncal valve overriding the VSD (Fig. 121–3A). The addition of Doppler interrogation will reveal truncal valve stenosis or regurgitation (see Fig. 121–3B). Suprasternal notch views can further define the anatomy of the pulmonary arteries and aortic arch (Fig. 121–4). Cardiac catheterization is generally reserved for the delineation of the anatomy in complex forms of truncus arteriosus, such as truncus arteriosus with a single pulmonary artery (Van Praagh type A3/B3). Cardiac catheterization is also indicated to assess PVR in the patient presenting late with truncus arteriosus. Magnetic resonance imaging (MRI) is a useful alternative or adjunct to cardiac catheterization for defining the anatomy of complex truncus arteriosus. MRI increasingly plays a role in the postoperative assessment of these patients in evaluating ventricular function as well as conduit and branch pulmonary artery anatomy.22
Natural History
The typical natural history of truncus arteriosus is characterized by early demise because of congestive heart failure. Approximately 40% of infants are dead within 1 month, 70% by 3 months, and 90% by 1 year.23 Those patients who survive infancy generally succumb by childhood or early adolescence because of congestive heart failure or, more commonly, pulmonary vascular obstructive disease. Rarely, patients survive infancy without developing pulmonary vascular obstructive disease, although those who do are estimated to be less than 5% of all patients.23
Treatment
Because of the inherent high early mortality, truncus arteriosus warrants early intervention. Initially, the surgical treatment of truncus arteriosus was limited to the banding of one or both of the branch pulmonary arteries. The first successful intracardiac repair was accomplished by Sloan’s group at the University of Michigan in 1962 using an unvalved polytetrafluoroethylene (PTFE) conduit for the pulmonary reconstruction.24 In 1967, McGoon and colleagues25 performed the first valved conduit repair, using an aortic allograft. During this period, complete repair was often undertaken as a staged procedure after initial pulmonary artery banding. However, complications of pulmonary artery banding, including pulmonary artery distortion, band migration, and failure to prevent the development of pulmonary vascular obstructive disease, resulted in a continued high mortality with this strategy. Ebert and coworkers26 published the first series of patients undergoing repair of truncus arteriosus in infancy in 1984. With continued improvements in neonatal operative techniques, as well as perioperative care, management has evolved to earlier complete repair. After the early reports of neonatal repair from the University of Michigan17 and the Children’s Hospital of Boston,27 neonatal repair has become the treatment of choice for truncus arteriosus.
Surgical Technique
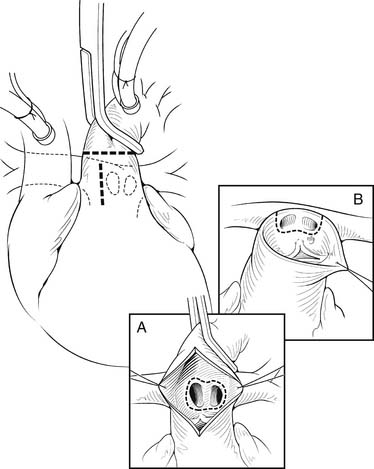
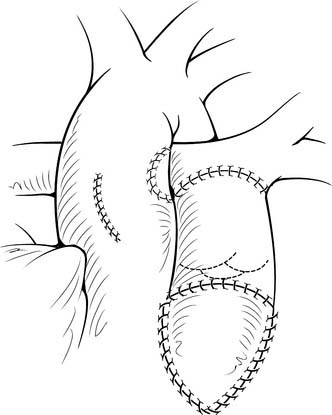
The heart is arrested with antegrade cardioplegia, with the aortic crossclamp placed as distally as possible. Significant truncal valve regurgitation may necessitate the use of retrograde cardioplegia. Once the cardioplegia is delivered, the pulmonary arteries are removed. If the coronary arteries can be positively identified and the exposure is adequate, the pulmonary arteries can be directly removed from the posterior aspect of the truncus, particularly for Collett and Edwards type I truncus arteriosus. The ostia of the coronary arteries can originate high in the sinuses or may have an intramural course.28 Direct assessment of the coronary anatomy in the aortic root is crucial if there is any uncertainty. Often, it is helpful to open the aorta anteriorly to aid in the removal of the pulmonary arteries and to identify the origins of the coronary arteries (Fig. 121–5A). Transection of the aorta just distal to the origins of the branch pulmonary arteries is another useful technique to facilitate pulmonary artery and coronary exposure (see Fig. 121–5B). The pulmonary arteries are removed, together with a small rim of adjacent truncal wall. Care is taken to avoid injuring the left coronary artery or truncal valve, which are often in close proximity to the pulmonary arteries. The defect is then repaired directly or with a patch of PTFE. At this point, additional doses of cardioplegia can be given at intervals, depending on surgeon’s preference.
The heart is inspected for the presence of any large conal branches, the location of the left anterior descending artery, and the possibility of aberrant coronary arteries. A right infundibulotomy is then made in an avascular area of the right ventricular outflow tract. The infundibulotomy is carried cranially toward the truncal valve and is limited to the size necessary to allow unobstructed output from the right ventricle through the anticipated conduit (Fig. 121–6A). Through the infundibulotomy, the VSD is closed with a patch of PTFE. We use a running suture technique, although an interrupted technique may be used as well. When there is membranous or inlet extension of the VSD, care is taken not to injure the conduction tissue in the posteroinferior aspect of the VSD. It is helpful to transition from the endocardium of the right ventricular outflow tract to the epicardium in the anterosuperior-most portion of the infundibulotomy, to avoid injuring the truncal valve and to ensure a widely patent left ventricular outflow tract (see Fig. 121–6B). If present, the atrial septal defect is then closed. A small PFO may be left as a “pop-off valve” to aid in the postoperative management.
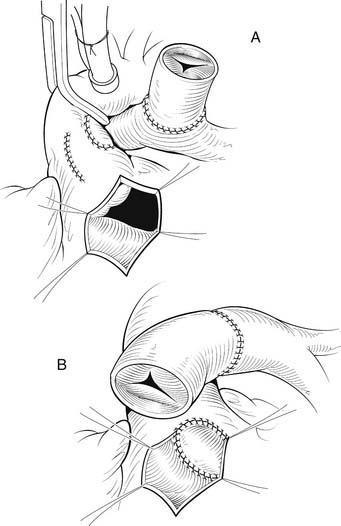
Once the VSD is closed, the remainder of the procedure may be performed with the crossclamp off and the heart beating. We prefer to use either bovine jugular vein grafts or cryopreserved pulmonary allograft for reconstruction of the pulmonary outflow tract, although heterograft valved conduit and cryopreserved aortic allograft are viable alternatives. The distal anastomosis of the allograft is performed to the confluence of the native branch pulmonary arteries. The posterior aspect of the allograft is then approximated to the superior portion of the right infundibulotomy. Anteriorly, the conduit is augmented with a patch of PTFE or additional allograft material (Fig. 121–7). A benefit of the bovine jugular vein grafts is the proximal extension of tissue, which can be used to patch the right infundibulotomy without additional patch material.
Other Surgical Considerations
Interrupted Aortic Arch
The surgical repair of truncus arteriosus with interrupted aortic arch (Fig. 121–8) requires several modifications to the basic operative procedure. The aortic cannulation is performed proximally at the base of the innominate artery to perfuse all of the brachiocephalic vessels, and distally in the ductus arteriosus to perfuse the distal aorta. Bicaval cannulation is used for venous return, with a left ventricular vent placed through the right upper pulmonary vein. The patient is placed on cardiopulmonary bypass and cooled to 18° C over a minimum of 20 minutes. The pulmonary arteries are snared to prevent pulmonary overcirculation. During cooling, the ascending aorta, arch, proximal descending aorta, brachiocephalic vessels, and pulmonary arteries are mobilized. The arch repair can be performed under deep hypothermic circulatory arrest. Alternatively, regional cerebral perfusion, as described by Pigula and coworkers29 and others, can be delivered by anastomosing a PTFE tube graft to the innominate artery or by directly cannulating the aorta at the base of the innominate artery and advancing the cannula into the innominate. After adequate cooling, the head vessels are snared, bypass is stopped, and regional cerebral perfusion is initiated, if desired. The ductus arteriosus is ligated just distal to the origin of the pulmonary arteries and divided. All residual ductal tissue is resected from the proximal descending aorta. The ascending aorta and proximal descending aorta are spatulated (Fig. 121–9). The back walls of the distal ascending aorta and proximal descending aorta are approximated, and the repair is augmented with a patch of cryopreserved pulmonary allograft (Fig. 121–10). Once the arch reconstruction is completed, the aorta may be recannulated and de-aired, an aortic crossclamp placed, and cardiopulmonary bypass resumed. Additional doses of cardioplegia may be administered and rewarming begun. The remainder of the repair, including VSD closure and pulmonary outflow tract reconstruction, is similar to the simple truncus arteriosus surgical procedure.
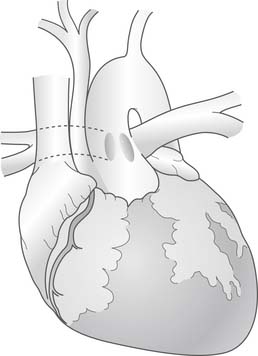
Figure 121–8 Truncus arteriosus (Collett and Edwards type II) with a type B interrupted aortic arch.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree