Treatment of Non–Small-Cell Lung Cancer: Surgery
Lung cancer represents the most common cause of cancer-related death in the world. It was considered a rare disease until the early part of the 20th century. In 1879, lung cancer represented only 1% of cancers identified at autopsy compared to 14% in 1927.1 The famous surgeon, Alton Oschner reported that as a medical student in 1910, he was called to the autopsy suite to see a case of lung cancer, since it was so rare he was likely to never see another case. Seventeen years passed before he saw another case of lung cancer and then he saw 8 cases in 6 months.2 The link between tobacco use and lung cancer has been common knowledge since the surgeon general’s report in 1964, and despite that knowledge, the death rate continued to rise in men until 1991 and in women until 2003. The treatment of lung cancer has evolved from a single modality, surgery, to a multimodality approach that calls upon the skills of numerous specialists. Physicians, who diagnose and treat lung cancer, must work together to define the role that surgery plays in the modern management of lung cancer. Surgery, when appropriate remains the cornerstone of therapy, and surgeons play an integral role in the diagnosis and treatment of patients with lung cancer.
DIAGNOSIS
The diagnosis and staging of lung cancer is covered elsewhere in the text. It is illustrative to consider the route taken by most patients before being referred to a surgeon and the qualifications that the surgeon should ideally possess to contribute optimally to the management of the patient with lung cancer. Patients with lung cancer may present with obvious symptoms such as hemoptysis or chest wall pain. The most common presenting complaints are respiratory symptoms that prompt a chest radiograph. From the surgeon’s viewpoint, the evaluation of patients with abnormal imaging studies depends on the likelihood of malignant disease. In patients with a history of smoking the level of suspicion is higher. Lung cancer is seen in nonsmokers, but the suspicion for cancer is significantly lower in this group than in smokers, in whom an abnormal chest radiograph is lung cancer until proved otherwise. If previous chest imaging is available those studies should be compared with the current imaging. A lesion that appears unchanged from older films, particularly those obtained more than 2 years previously, markedly diminishes, but does not eliminate, the probability that the current finding represents a lung cancer.
When a surgical referral is indicated, the surgical specialist should offer a complete armamentarium of surgical options and techniques as well as an understanding of the principles of lung cancer treatment. The technical ability to perform lung resection is the minimum requirement but by itself is not sufficient. There is an increasing trend toward thoracoscopic techniques such as video-assisted thoracic surgery (VATS) or robot-assisted surgery. At least 30% of lobectomies are performed using minimally invasive techniques but this proportion is rising rapidly.3 Thoracoscopic lung resection has been shown to be associated with a decreased length of stay, decreased complication rate, and decreased need for blood transfusion.4,5 The lymph node harvest is equivalent if not superior.3 The thoracic surgical specialist should be proficient in staging procedures including mediastinoscopy and endobronchial ultrasound (EBUS) although in many hospitals, a pulmonologist performs this latter procedure. The critical element is the willingness of the surgical specialist to participate in coordinated multidisciplinary care. Patients with advanced disease, who may be candidates for resection after induction therapy, should be referred early in the process so that decisions about potential fitness for operation from oncologic, medical, and technical perspectives can be made.
As critical care and anesthetic techniques continue to improve and minimally invasive surgery advances, all patients with lung cancer should be considered for therapy by the treating specialists. Age bias against treatment including chemotherapy, radiation, and surgery has been documented across many fields of medicine.6 Age alone should not be the basis for ruling out definitive treatment and each patient must be evaluated individually.
Patients may present with symptoms either referable to the chest or related to the presence of metastatic disease. The initial evaluation should be directed toward an explanation of the symptoms. A complete discussion of the clinical presentation is available in Chapter 112. Patients with evidence of metastatic disease still require a tissue diagnosis. The method employed to obtain the tissue diagnosis should have the highest probability of success. Whereas bronchoscopy has a high likelihood of yielding a diagnosis in the patient who presents with cough or hemoptysis and a central lesion, it is less likely to be successful when the lung findings are confined to a peripheral small nodule. In these cases, transthoracic needle biopsy may have the highest yield. When a patient presents with presumed metastatic disease that is accessible, such as a palpable supraclavicular lymph node, a fine-needle aspirate, done in the office, may yield the diagnosis and obviate the need for mediastinal staging. Consideration must be given to tissue yield, since ideally a biopsy sample should have sufficient tissue to allow for the ever-expanding array of genetic testing such as for EGFR mutations or ALK gene rearrangements. Some patients with metastatic disease may require wedge resection or other excisional biopsy to obtain enough tissue to allow for extensive molecular testing. A percutaneous biopsy of an adrenal lesion may also provide both a diagnosis and stage; however, prior to biopsy, the likelihood of pheochromocytoma should be considered. Previously the only question of significance in the patient with metastatic disease was the differentiation between small cell and non–small-cell lung cancer (NSCLC). However, current adjuvant or definitive treatment algorithms differ considerably between the different histologic types of NSCLC. Occasionally mediastinoscopy may be required to obtain enough tissue and very rarely video thoracoscopic excision of a lung nodule may be needed. Exploratory thoracoscopy or thoracotomy has no place in the management of these patients.
For patients who present with a solitary pulmonary nodule, chest computed tomography (CT) should be performed to determine if the nodule is solitary as well as to assess the status of the mediastinum, liver, and adrenal glands. When the risk of malignancy is low, such as in nonsmokers with a small nodule, it is reasonable to follow the lesion over a period of time. A repeat chest CT in 3 to 12 months, depending on the size of the nodule, is the recommended approach in the low-risk candidate. If the lesion has increased in size, then excision is carried out. If there is no change in size continued observation with repeated chest imaging is warranted. Conversely, in a smoker in whom there is a high probability that the nodule is malignant, excision may be justified without a prior needle biopsy.
The role of percutaneous needle biopsy of the solitary pulmonary nodule remains controversial. Diagnostic accuracy of CT-guided biopsy has been reported as high as 93.5% but this is due largely to its ability to diagnose malignant as opposed to benign processes.7 The accuracy can be improved with increasing use of core needles and on-site cytologic analysis. In a patient at high risk for cancer, a positive biopsy confirms what we already suspected; however, the patient has been exposed to the risk of the needle biopsy, namely, a 10% to 15% incidence of pneumothorax with a need for a chest tube in 2% to 5% of cases.8 The National Lung Screening Trial reported a 21.2% complication rate of needle biopsy with one death.9 A negative biopsy does not negate the fact that a suspicious nodule remains and is of little help. More definitive information has to be obtained, such as cartilage or fungal elements, for the biopsy to obviate the need for resection in a high-risk patient with a suspicious lesion. If biopsy is inconclusive or the patient has significant anxiety regarding the possibility of a pulmonary nodule being an occult cancer, then resection may be indicated. Most peripheral lesions can be resected thoracoscopically and sent for frozen section. If the nodule proves to be benign, then thoracoscopic excision both makes the diagnosis and completes the therapy. If the nodule is malignant, an anatomic or other therapeutic resection and lymph node sampling can be performed during the same operative session. A biopsy consistent with small cell cancer should not preclude resection if there is no mediastinal adenopathy. T1-2N0-1 small cell lung cancers are optimally treated with anatomic resection and mediastinal lymph node dissection or sampling.10 Most thoracic surgeons would perform mediastinoscopy and/or EBUS prior to resection to confirm that the mediastinum is not involved with tumor. If the final pathology reveals small cell carcinoma, resection is followed by chemotherapy.
STAGING
A discussion of noninvasive staging is beyond the scope of this chapter and is dealt with elsewhere in this volume; however, the role of invasive staging and specific procedures utilized deserve mention. Staging determines the appropriate surgical and medical treatment plan. Therefore, thorough staging is mandated. In discussing stage, distinction must be made between clinical and pathologic stage. The former is based solely on noninvasive imaging studies, whereas the latter depends on actual histologic material obtained either by invasive staging studies or at the time of the surgical resection. The accuracy of clinical staging depends upon the accuracy of the study. A chest CT scan provides excellent visualization of the contents of the superior mediastinum. Typically, mediastinal lymph nodes are considered suspicious if they are greater than 1 cm in short axis. However the CT must always be considered in context of the remainder of the mediastinum. Volterrani et al.11 have suggested creating size cutoffs for each of the mediastinal lymph node stations to improve diagnostic accuracy of CT alone. Fused CT-PET provides information on the metabolic activity of the primary lesion and the mediastinal lymph nodes and may detect occult distant metastases. A recent study in patients with clinical stage I NSCLC, who underwent staging with PET followed by resection and lymphadenectomy revealed the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of PET to be 68%, 95%, 31%, 99%, and 94% respectively.12 Thus PET is a highly specific test. However, a patient without obvious distant metastatic disease and a PET or CT that is concerning for mediastinal metastasis should not be deemed inoperable based on imaging alone. Patients with granulomatous lymphadenitis from sarcoidosis or histoplasmosis frequently have hypermetabolic lymph nodes not due to malignancy. These patients should undergo invasive mediastinal staging. In addition, patients with postobstructive pneumonia often have enlarged or hypermetabolic mediastinal lymph nodes as a reaction to infection. At our institution, in these cases, we perform EBUS and if negative for malignancy, we perform mediastinoscopy at the time of planned resection. EBUS followed by mediastinoscopy provides a greater sensitivity than either test alone and helps eliminate sampling error.13 When performing EBUS for staging and diagnosis a logical progression from N3 to N2 to N1 nodes should be followed, minimizing the need for sampling nodal stations that would not affect the stage and preventing upstaging through needle contamination.14
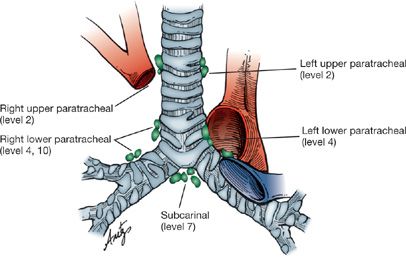
With the constantly improving resolution of CT imaging and the increasing availability of EBUS, the need for routine mediastinoscopy for all patients with lung cancer must be questioned. While still considered a definitive staging technique, there are nodal stations that cannot be accessed by standard cervical mediastinoscopy. These include the subaortic nodes (level 5) and para-aortic nodes (level 6), both of which are located in the aortopulmonary window (Fig. 113-1). They are a common site for lymph node involvement in left upper lobe tumors but when involved in the absence of other lymph node disease, the prognosis is equivalent to N1 (hilar) disease. These nodes are not accessible through standard EBUS techniques and are difficult to access via the esophagus although transarterial sampling has been described but is rarely performed.15
Figure 113-1 Lymph node stations accessible by cervical mediastinoscopy. For consistency the levels should be labeled with the appropriate number when submitted to surgical pathology.
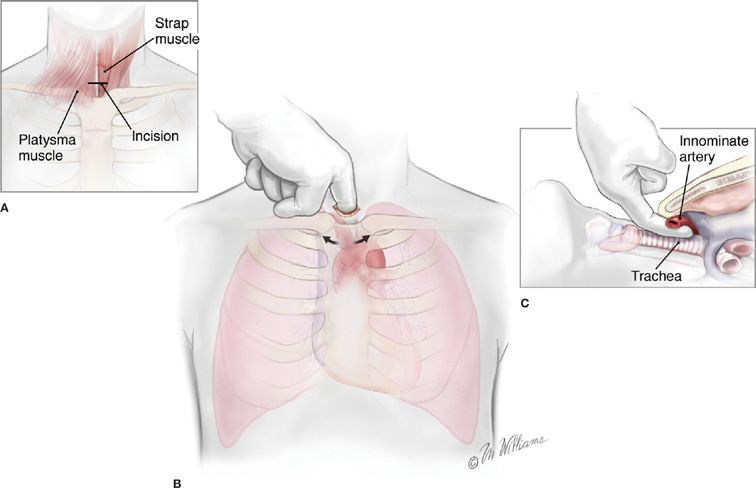
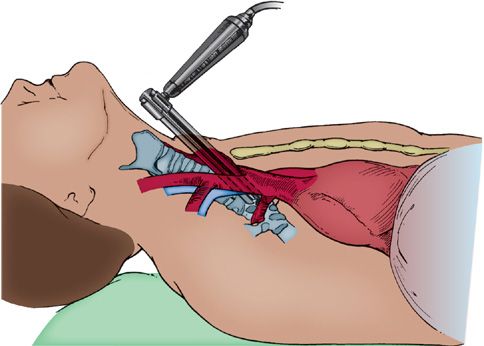
Mediastinoscopy is performed through a small (2.5-cm) incision made in the neck 1 cm above the sternal notch. The area explored by mediastinoscopy, the superior mediastinum, is palpated first by inserting a finger along the anterior aspect of the trachea, which also serves to develop the space and facilitate insertion of the mediastinoscope (Figs. 113-2 and 113-3). Obviously involved lymph nodes often may be palpated, but palpation alone is insufficient, since microscopic disease may be present that can only be identified if representative biopsies of the important nodal stations are taken following insertion of the mediastinoscope. Visual inspection without histologic confirmation is also to be scrupulously avoided. Of major importance are the ipsilateral nodes, but just as important is the status of the contralateral lymph nodes, especially in left lower lobe lesions, in which right paratracheal lymph nodes are commonly involved. Despite notions to the contrary, left-sided lymph nodes are readily accessible at mediastinoscopy but are somewhat more difficult to identify. In fact the left paratracheal lymph nodes are much more easily sampled at mediastinoscopy than at left thoracotomy because of the location of the aortic arch relative to the left mainstem bronchus. Because of this we have a much lower threshold for performing mediastinoscopy for left-sided lesions. Nodal stations most frequently sampled include levels 2 (upper paratracheal) and 4 (lower paratracheal) on the right, level 3 (pretracheal), level 7 (subcarinal), and level 4 on the left. Because the left level 4 lymph nodes occur at a slightly higher location, identifying separate level 2 nodes on the left can be difficult. It is not necessary always to sample all nodal stations; if there are nodes obviously involved, these, along with contralateral nodes, are all that are necessary to adequately stage the patient.
Figure 113-2 Technique of cervical mediastinoscopy. A. A 3-cm incision in the midline one fingerbreadth above the sternal notch. B. Pretracheal tunnel fashioned with blunt dissection using the index finger. C. Dorsal aspect of the finger remains on the trachea while the volar aspect comes in contact with the innominate artery. (Used with permission of Marcia Williams.)
Figure 113-3 Mediastinoscope in place demonstrating the superior mediastinal plane.
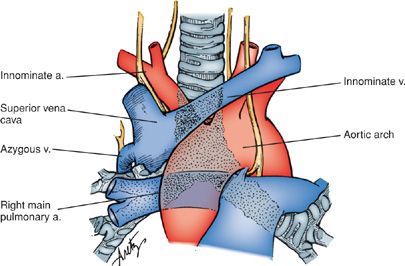
Mediastinoscopy is a technically demanding procedure that requires significant training to perform correctly. The close proximity of a number of major vascular structures makes it daunting even to the experienced practitioner. Major vessels within the operative field and therefore at risk include the innominate artery, aortic arch, superior vena cava, azygous vein, and right main pulmonary artery (Fig. 113-4). The left recurrent laryngeal nerve and esophagus are also subject to injury. A recent meta-analysis of the complications of mediastinoscopy reported a morbidity of 0% to 5.3% and a mortality of 0% to 0.05%.16 Despite the stakes of the procedure, in experienced hands, the actual risk of the procedure is quite low and all but the most debilitated and infirm patients tolerate the procedure well. Inability or reluctance to adequately stage the mediastinum may result in unnecessary or unsuccessful thoracotomy or thoracoscopy.
Figure 113-4 The relationship of major vascular structures potentially encountered during mediastinoscopy to the trachea and main bronchi.
Although mediastinoscopy is the mainstay of invasive staging for lung cancer, other procedures provide additional information that often complements that obtained at mediastinoscopy. The aortopulmonary window, a common site of nodal spread from tumors of the left upper lobe, and one that cannot be reached with conventional mediastinoscopy or EBUS without traversing the left main pulmonary artery, may be reached with an anterior mediastinotomy, also known as the Chamberlain procedure. An incision is made over the left second costal cartilage, the cartilage is excised, and the pleural reflection is swept laterally to access the aortopulmonary window in an extrapleural plane. Typically, anterior mediastinotomy is performed in conjunction with cervical mediastinoscopy, and the mediastinoscope is used for visualization. Tube thoracostomy is rarely needed and this procedure can be performed as an outpatient. The involvement of lymph nodes at this level (level 5) in the absence of other nodal disease is associated with a 5-year survival that approaches 50% if the disease can be completely resected, a survival that is almost identical to that seen with N1 (hilar) disease. The rationale, then, behind performing parasternal mediastinotomy either is to assess resectability or document mediastinal nodal disease to justify placing the patient into an experimental protocol of neoadjuvant chemotherapy, radiation therapy, or both.
Similarly, video-assisted thoracoscopic surgery (VATS) aids in the staging of lung cancer, as an occasional adjunct to, but not in lieu of, mediastinoscopy. VATS offers an opportunity to sample nodes on the right and left through one incision and provides access to aortopulmonary window (level 5), ascending aortic (level 6), paraesophageal (level 8), and inferior pulmonary ligament (level 9) nodal stations. It is notable that levels 4R, 2R, 7, 8, and 9 nodes are often easily biopsied via esophageal endoscopic ultrasound. VATS also visualizes the pleural space, especially useful in the patient with a pleural effusion and negative fluid cytology, so as to rule out diffuse pleural involvement and prevent an unnecessary thoracotomy. Other nodules seen on CT scan that may have an impact on treatment planning also may be excised and defined histologically prior to formal thoracotomy.
Usually the ultimate decision regarding resectability of a locally invasive lesion must be made at the time of thoracotomy when the lesion itself may be palpated and the dissection conducted under greater control. Direct invasion into mediastinal structures including the superior vena cava or vertebral bodies is not a contraindication to resection. As imaging has evolved, the rate of exploratory thoracotomies has dropped. A logical progression from the less invasive to the more invasive procedures guided by the imaging studies often results in the patient being spared a procedure from which there will be no significant benefit.
SURGICAL TREATMENT OF LUNG CANCER
When evaluating a patient with lung cancer for possible resection there are three different criteria that must be satisfied: (1) Is resection oncologically beneficial, (2) is resection technically feasible, and (3) does the patient have the physiologic fitness to tolerate resection?
Staging studies and procedures determine the oncologic benefit of therapy. The feasibility of resection is typically a decision made by the operating surgeon. Involvement of ribs, vertebral bodies, or mainstem bronchi does not preclude resection. Extended resections are discussed later in this chapter. The final and often most difficult criterion to satisfy is the patient’s physiologic fitness to undergo surgery. Factors that must be considered include pulmonary reserve, exercise capacity, heart disease, and other medical comorbidities. Frailty indices can be calculated and correlate with postoperative complications.17 Cardiac risk factors should be identified and evaluated per published guidelines.18 Often borderline patients benefit from functional testing such as stair climbing or shuttle walking. These tests are easily performed in the office and are associated with minimal cost. The height climbed or speed at which the patient climbs a set number of stairs can predict postoperative complications.18,19 Formal exercise testing with measurement of oxygen consumption may help quantify the risk of lung resection in selected patients. Quantitative perfusion lung scans have allowed us to better select borderline patients for pulmonary resection, especially when pneumonectomy is a possibility. This information has all but eliminated the “pulmonary cripple” as a result of a lung resection. Quantitative split perfusion testing may identify poorly perfused lung tissue, especially in the setting of upper lobe tumors surrounded by severe upper lobe predominant emphysema. Those patients may tolerate resection because of a volume reduction effect. Experience with lung transplantation has shown that deconditioned patients benefit from at least a 6-week period of pulmonary rehabilitation, and selected patients with otherwise operable disease may be placed in a rehabilitation program before undergoing surgery.20 Smoking cessation is of paramount importance.
Thoughtful and thorough evaluations combined with less invasive surgical approaches have allowed many patients who previously were not considered surgical candidates to undergo surgery. All but the frailest patients should be referred to a thoracic surgeon to allow this determination.
In spite of recent advances in nonoperative therapy, surgery remains the best treatment for early-stage disease. It is important that the appropriate procedure be performed. The types of resection include anatomic resection such as segmentectomy, lobectomy, bilobectomy, and pneumonectomy; and nonanatomic wedge resections. Anatomic resections involve the individual ligation of the feeding pulmonary artery, vein, and bronchus to the region of the lung to be resected. Wedge resections are nonanatomic operations where lung parenchyma is divided without ligation of the feeding vasculature or bronchi. Extended resections include an anatomic resection with associated structures that are involved with the cancer. These can include portions of the chest wall resected en bloc, vertebral bodies, diaphragm, pericardium, left atrium, or superior vena cava. Also included in extended resections are bronchoplastic resections or pulmonary artery reconstruction procedures. The choice of resection is multifactorial based on the location and stage of the tumor and the patient’s physiologic fitness.
 OPEN VERSUS MINIMALLY INVASIVE TECHNIQUES
OPEN VERSUS MINIMALLY INVASIVE TECHNIQUES
The role of minimally or less invasive surgical techniques in lung cancer resection continues to evolve. Currently most pulmonary resections are performed by thoracotomy but an increasing number of thoracic surgeons now perform these oncologic procedures using VATS or robotically assisted resections. VATS is analogous to laparoscopic surgery wherein operative visualization is provided by an endoscopic camera as opposed to direct visualization through the incision. Currently at least 30% of lobectomies for lung cancer are performed using VATS.21 It should be emphasized that although the approach is different, the principles of anatomic lung resection should remain the same. Anatomic lung resections most typically performed by VATS include lobectomy and segmentectomy. Pneumonectomy has been reported as well as bronchoplastic procedures.22,23 The key elements of these procedures involve the identification and individual ligation of the relevant branches of the pulmonary artery, pulmonary veins, and bronchi. A thorough mediastinal lymph node sampling should also be performed. In experienced hands, VATS lymph node harvest is equivalent when compared to thoracotomy.24,25 The perioperative outcomes have been superior to thoracotomy for quality of life and pain.26 Multiple studies have demonstrated oncologic equivalency, decreased complication rate, and decreased levels of inflammatory mediators.27,28 Minimally invasive approaches to lobectomy are associated with decreased pain and length of stay.28,29 There is increasing evidence that patients who undergo VATS lobectomy are better able to tolerate adjuvant chemotherapy and receive a greater proportion of the planned dose.26,30 Robotic-assisted surgery continues to evolve; multiple case series have been reported and a retrospective multi-institutional review of robotic lobectomy revealed perioperative and long-term outcomes to be equivalent to VATS lobectomy.31 Although VATS techniques are associated with improved outcomes in selected patients, many patients are best treated with open surgery. The decision of whether to employ a minimally invasive technique versus thoracotomy depends on many factors such as tumor size and location, extent of chest wall involvement, the presence of calcified lymphadenopathy, and surgeon experience.
 TYPE OF RESECTION
TYPE OF RESECTION
Surgical considerations in management of lung cancer include lobectomy versus a lung-sparing resection.
Lobectomy
Lobectomy remains the definitive resection for most lung cancers, since it is an anatomic resection that removes the regional lymph nodes located along the lobar bronchus. Other choices include limited resections such as wedge resection, and segmentectomy. Some hilar tumors or those involving the chest wall or other structures may require pneumonectomy or extended resections. Doing less than a lobectomy may represent a compromise, although a nonanatomic wedge excision may be considered for small primary tumors. Tumors located within the center of the lobe or near the hilum typically require lobectomy.
Limited Resection: Wedge Resection and Segmentectomy
Smaller tumors that are peripherally situated may be candidates for lesser resections. Only patients with clinical stage I cancer (T1N0, T2N0) are possible candidates for a curative limited or sublobar resection. Tumor characteristics and location also play an important role in decision making. Pure ground-glass opacities on CT scan often are revealed to be adenocarcinoma in situ, adenocarcinoma with lepidic growth pattern, and minimally invasive adenocarcinoma with pure lepidic growth; this group of tumors was formerly known as bronchioloalveolar carcinoma (BAC). These lesions, if small, can be considered for limited resection, since resection yields almost 100% survival and may have equivalent survival after wedge resection.32,33 Combining CT and PET characteristics such as percent ground-glass opacity and standardized uptake value (SUV) can aide in predicting who would benefit from limited resection.34
Wedge resection crosses lymphatic channels, does not remove the originating bronchus, and does not permit adequate regional lymph node resection, and parenchymal margins may be limited. These factors limit the value of wedge resection as a planned curative procedure for most primary lung cancers. Wedge resection is, at best, a compromise, and patients who otherwise could tolerate an anatomic resection are not well served by having less done. External beam radiation has been used in an attempt to augment the efficacy of wedge resection but it has been associated with increased pulmonary toxicity in a patient population that typically has compromised pulmonary function. Several groups have reported decreased local recurrence with adjuvant brachytherapy at the time of resection. 125I seeds embedded in an absorbable mesh are applied to the line of resection.35 A randomized phase III trial comparing sublobar resection with and without brachytherapy (ACOSOG Z4032) in high-risk patients has completed enrollment but the results are still maturing at the time of publication. Perioperative morbidity and mortality were not increased in patients receiving brachytherapy.36
Segmentectomy represents a more favorable option for limited resection than wedge resection. As an anatomic resection, segmentectomy provides a wider parenchymal margin and encompasses the regional lymphatics, which drain centrally, and permits regional lymph nodes to be evaluated during the resection. Parenchymal margins of resection are identified more clearly when an anatomic resection is accomplished and a longer length of bronchus is removed, minimizing the opportunity for local bronchial recurrence. Anatomic segmentectomy is the operation of choice in patients with marginal pulmonary function because mortality is lower, and long-term survival is almost comparable to lobectomy.
Segmentectomies are often more technically demanding to perform compared to lobectomy. Many surgeons who do not perform a high volume of pulmonary surgery may not possess the expertise required to perform this procedure. Segmentectomy can be performed by open or VATS techniques. The prototype segmental resection is the superior segment of the lower lobe, but any lung segment may be removed anatomically. Typically the basilar segments of either lower lobe are resected as a unit. The lingular segments or the upper division of the left upper lobe are often resected together. Segmentectomy of the middle lobe is rarely performed.
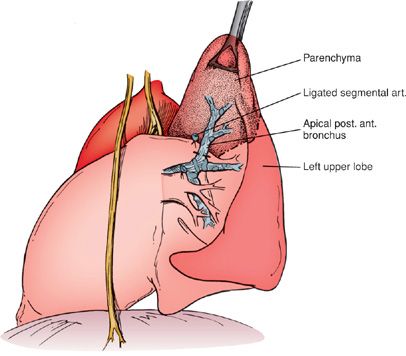
The key to segmental resection is the identification of the segmental artery, which, once ligated and divided, reveals the location of the segmental bronchus that is taken next. The segmental vein is divided last, and the parenchyma is divided with a stapler or stripped by dividing the lung parenchyma in the intersegmental plane using a combination of blunt and sharp dissection (Fig. 113-5). When properly performed, this technique is not associated with significant blood loss or air leak.
Figure 113-5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree