Treatment of Non–Small-Cell Lung Cancer: Radiation Therapy
Lung cancer is the second most common malignancy in the United States, after prostate cancer among men and breast cancer among women, but the number one cause of cancer-related mortality in both genders. Non–small-cell lung cancer (NSCLC) accounts for 80% to 85% of all lung cancers.1 Most patients with NSCLC receive radiotherapy as part of their treatment, either as initial management or later in the course of their disease. This may include thoracic radiotherapy and/or irradiation of sites of metastatic disease.
Thoracic radiotherapy for NSCLC can be categorized as follows:
• Neoadjuvant = preoperative
• Adjuvant = postoperative
• Definitive = cure without surgery as treatment goal; with or without chemotherapy
• Palliative = directed at relief of thoracic symptoms
There is some overlap in these categories with respect to the goals of treatment. For example, most patients treated with definitive intent are not cured but do achieve palliation of thoracic symptoms. Similarly, a few patients originally considered to be technically unresectable may have a dramatic response to irradiation and/or chemotherapy, and the goal of treatment may then change from palliative to neoadjuvant or definitive intent.
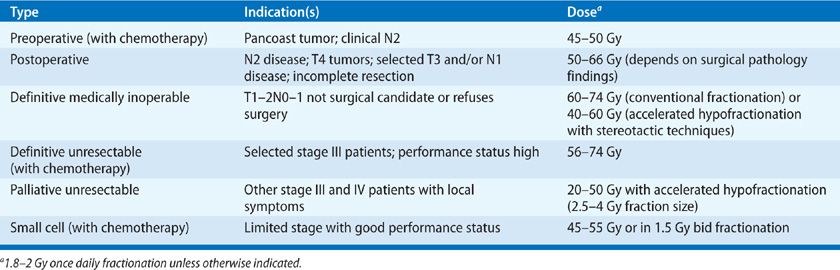
The size of the primary lesion, stage, and total dose of radiation are important factors in determining the likelihood of achieving local control. A summary of radiotherapy for lung cancer is provided in Table 115-1.
The decision to utilize thoracic radiotherapy as part of the therapeutic regimen, and the treatment goals of such therapy, depend not only on tumor-related factors such as stage but also on patient-related factors such as pulmonary reserve and performance status. All these factors need to be considered when deciding whether to irradiate. Although radiotherapy might be appropriate for a patient with a postoperative forced expiratory volume (FEV1) of 2 L and pathologic stage T2N2M0 disease, the same treatment would be problematic in a patient with a postlobectomy FEV1 of 1.1 L who has had a series of postoperative complications. Of course, such clear-cut cases usually are the exception rather than the rule in clinical oncology. Table 115-2 lists the relative contraindications to thoracic radiation for lung cancer.
Finally, it must be remembered that the prognosis for most patients with lung carcinoma remains poor with standard therapy, and a concerted effort should be made to enter patients into clinical trials that are investigating new treatments or combinations of treatments for this disease.
THORACIC RADIOTHERAPY MANAGEMENT STRATEGIES
Surgery, irradiation, and chemotherapy are all used in the treatment of NSCLC and are based on the stage of the disease at presentation. Staging for all patients should include PET-CT scan, and selected patients should have bronchoscopy with endobronchial ultrasound (EBUS)-guided sampling of intrathoracic lymph nodes or mediastinoscopy, and MRI of the brain. Surgical resection remains the primary curative modality and may be the only treatment required in early-stage disease. The local failure rate in stage I patients after lobectomy or pneumonectomy is less than 10%. With such a low incidence of local failure the addition of postoperative irradiation is unnecessary if resection margins are negative. Unfortunately, many patients present with locally advanced, unresectable, or marginally resectable disease. In marginally resectable NSCLC, the addition of radiation and chemotherapy is aimed at decreasing the high frequency of failure due to local and distant spread that occurs with surgery alone. However, progress has been slow, and the overall survival of patients with locally advanced lung cancer has only increased modestly over the past 20 years.
 NEOADJUVANT THERAPY
NEOADJUVANT THERAPY
The current use of preoperative radiotherapy in the management of NSCLC falls into two categories: (1) as part of neoadjuvant chemoradiotherapy for N2 (IIIA) disease; and (2) as part of neoadjuvant chemoradiotherapy for superior sulcus (Pancoast) tumors (T3-4NxM0). For patients who are otherwise surgical candidates but are found at mediastinoscopy to have positive mediastinal lymph nodes (N2 disease), it is not clear whether neoadjuvant chemoradiotherapy is superior to neoadjuvant chemotherapy; either is an acceptable option.
N2 Disease
Conceptually, it has become attractive to attempt to convert bulky mediastinal nodal disease to microscopic mediastinal nodal disease, thereby rendering the patient suitable for surgical resection. Two large randomized trials were conducted to compare surgical versus nonsurgical management for highly selected patients with stage III disease.2,3 These studies demonstrated the safety and feasibility of the surgical approach, but no definitive improvement in survival by adding surgery to chemoradiotherapy. However, secondary analyses suggested a favorable outcome in patients who had a pathologic complete response after neoadjuvant therapy. Patients undergoing lobectomy after neoadjuvant therapy appear to have better outcomes than patients who require pneumonectomy. Despite several large phase III randomized trials, the optimal approach remains unclear. Options include chemotherapy followed by surgery, chemoradiotherapy followed by surgery, and definitive chemoradiotherapy alone. The median and 5-year survival rates for these three options appear to be similar. There is evidence that induction chemotherapy or chemoradiotherapy followed by pneumonectomy (particularly right pneumonectomy) may be excessively toxic, with the previously mentioned studies reporting treatment-related mortality rates above 20%. In contrast, induction therapy (chemotherapy or chemoradiotherapy) followed by lobectomy appears to be well tolerated.2–4
Chemoradiotherapy followed by thoracotomy is an intensive treatment with considerable morbidity and mortality. Its use should be limited to patients with excellent cardiac and pulmonary reserve and a high performance status. Preferably this combination should be used in the context of a prospective clinical trial. Only patients with reasonable expectation of benefit should receive this form of aggressive management; thorough staging workups for metastatic disease should be performed prior to the start of preoperative treatment and in the “window” period (i.e., after this therapy has been administered and before surgery). Lesions that are suspected of being distant metastases should be investigated by tissue biopsy. The radiotherapy dose should be moderate, approximately 45 to 50 Gy, with standard fractionation (1.8–2 Gy once daily). An interval of approximately 3 to 8 weeks between completion of irradiation and surgery is advised to minimize the risk of difficulties in wound healing. Bronchial stump reinforcement at the time of surgery is strongly encouraged. As noted, right pneumonectomy after neoadjuvant chemoradiotherapy has a high mortality rate and should be avoided.
Preoperative radiotherapy carries with it the potential disadvantage of limiting the ability to give additional radiotherapy if the tumor proves to be unresectable or if residual disease remains after resection. After 45 Gy preoperatively, only about 30 Gy of additional irradiation can be safely administered postoperatively. Thus, it may be preferable to defer additional radiotherapy unless or until there is clear evidence of local progression. Chemotherapy may be offered, although in general, the patient left with residual or unresectable disease after preoperative chemoradiotherapy has a low likelihood of achieving long-term disease-free survival.
Superior Sulcus (Pancoast) Tumor
Superior sulcus tumors are uncommon lung cancers and account for only 3% of all lung cancer cases. They deserve special consideration as they can invade blood vessels if located anteriorly, brachial plexus if located in the middle or the stellate ganglion, or vertebral bodies if located posteriorly. These syndromes can cause extremely debilitating symptoms that are difficult to manage. In addition to the routine staging workup, MRI scan of the chest and/or spine may be very valuable to rule out any brachial plexus or vertebral body invasion.
Treatment depends on the extent of the disease. Presence of hilar or mediastinal nodal involvement is associated with poorer outcome. Single center studies have reported improved outcome using preoperative radiotherapy with or without chemotherapy. A phase II study by SWOG (SWOG 9416/INT0160) showed 2-year overall survival of 55% in T3–4N0 superior sulcus tumors.5 In most institutions, preoperative chemoradiotherapy is standard management for superior sulcus tumors, with excellent outcome. However, selected patients may undergo surgery first followed by adjuvant treatment. In patients who are not candidate for surgical resection, concurrent chemoradiation is the preferred treatment approach.
 ADJUVANT THERAPY
ADJUVANT THERAPY
The primary tumor-related factors considered in decisions about the need for postoperative radiotherapy (PORT) are the pathologic stage and the completeness of the surgical resection. PORT is generally considered to be the standard of care for patients with resected ipsilateral mediastinal node–positive (N2) NSCLC, based in part on a Lung Cancer Study Group Trial that demonstrated a longer relapse-free survival with PORT in this subgroup.6 There is no role for PORT for T1-2N0 tumors completely resected by lobectomy or pneumonectomy. The role of PORT for T1-2N1 tumors is questionable; in fact, there is the suggestion of a slight detrimental effect of PORT on overall survival. It is less clear whether radiotherapy should be administered after a wedge resection, although in selected patients, the high local failure rate after this procedure suggests a possible role for adjuvant radiotherapy.7 Phase II data suggest that the combination of wedge resection plus brachytherapy results in local failure rates below 5%, comparable to that of lobectomy, and this approach can be considered in this situation. A multicenter phase III trial suggested sublobar resection with brachytherapy is feasible without any increase in morbidity compared to sublobar resection alone, and with low mortality at 30 and 90 days in high-risk patients with NSCLC.8
Whether PORT for stage IIIA NSCLC (N2-positive disease) has any impact on survival is debatable. Although many retrospective studies have shown a survival benefit to PORT, prospective, randomized trials have not. In fact, a highly publicized meta-analysis of randomized trials of surgery alone versus surgery plus PORT showed a detrimental effect of PORT on survival for lower stage.9 This negative effect of PORT was very strong for stage I disease and modest for stage II disease; there was no evidence of any detrimental effect of PORT in stage III disease. The reasons for excess deaths in the PORT arm were not addressed, though likely attributable to radiation-induced cardiopulmonary toxicity. It should be noted that the legacy randomized trials included in the PORT meta-analysis and also in the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute all used radiotherapy techniques that would be considered outdated by modern standards.
A more recent study utilizing the SEER database looked at the role of PORT in resected NSCLC. There was no difference in survival for the overall group but a subgroup analysis suggested increased survival with PORT in the N2 subgroup and decreased survival in N0–1 subgroups.10 Douillard et al.11 retrospectively analyzed patients in the Adjuvant Navelbine International Trialist Association (ANITA) trial, and found a benefit to PORT in patients with IIIA/N2 disease.
Future studies of PORT should focus on stage III disease and selected stage II disease, and on coordinating selective use of PORT with adjuvant chemotherapy. The majority of patients with node-positive resected NSCLC probably harbor micrometastatic disease outside of the thorax and thus adjuvant chemotherapy is appropriate. However, the risk for potentially morbid local–regional recurrence also exists in these patients. Further research is needed to better identify which patients are at very high risk for local–regional recurrence and thus most likely to benefit from PORT. If more effective therapy to prevent distant disease is developed, then the improvement in local control may lead to consistent significant increases in survival.
When employing PORT, meticulous radiotherapy treatment planning is essential to minimize risks of toxicity. Radiotherapy fields should be relatively modest in size, yet include the high-risk regions of the bronchial stump, ipsilateral hilum, and the portion(s) of the mediastinum considered high risk for regional recurrence. If resection margins are negative and if there is no chest wall invasion, there is no reason to irradiate the “tumor bed”; doing so would only increase toxicity by irradiating that portion of remaining lung that has filled into the space left by the lobectomy. A radiation dose of 50 to 55 Gy using a standard fractionation schedule (1.8–2 Gy per day) should provide excellent local and regional control. Higher doses may be reasonable if resection margins are compromised and the patient has excellent underlying cardiopulmonary function.
Patients who undergo incomplete resection (gross residual disease) or suffer local recurrence after surgery alone have a poor prognosis, although radiation is usually used in an attempt to maximize local control. These patients should be considered to have the equivalent of locally advanced, nonoperative NSCLC and treated accordingly, potentially with definitive intent (see the following).
 DEFINITIVE THERAPY (LOCALLY ADVANCED, NONOPERATIVE NON–SMALL-CELL LUNG CANCER IIIA OR IIIB)
DEFINITIVE THERAPY (LOCALLY ADVANCED, NONOPERATIVE NON–SMALL-CELL LUNG CANCER IIIA OR IIIB)
Patients who do not have demonstrable distant metastases but have locally advanced, unresectable disease are often referred for radiation therapy, with or without chemotherapy. For nonoperable (stage IIIA and IIIB) patients, combined chemotherapy and radiation therapy is often considered as the standard of care in patients with good performance status and no other significant medical comorbidities.12 Because combined modality therapy has considerable toxicity and requires a high level of patient time, commitment, and expense, intensive chemoradiotherapy generally should be limited to patients whose Karnofsky scores are 70% or greater. Significant weight loss, defined in most cooperative group trials as greater than 5%, is also a relative contraindication to aggressive combined modality therapy as it carries a poor prognosis. Although age itself is not a contraindication to combination therapy, intensive regimens should be applied cautiously in patients more than 70 years old. Notably, the median age averaged 60 years in most of the trials utilizing chemoradiotherapy. Although large tumor size is not a contraindication to definitive treatment, larger tumors generally result in a larger portion of normal tissue (lung, heart, and esophagus) being included in a radiotherapy portal, and the resultant high-dose irradiation may carry an unacceptable risk of complications. The location of the tumor (e.g., proximity to the heart and/or extensive involvement of the right lower lobe of the lung) and the patient’s pulmonary reserve may also influence the decision regarding definitive irradiation. Supraclavicular adenopathy (N3 disease) is not an absolute contraindication for definitive therapy, although its presence is a poor prognostic indicator.
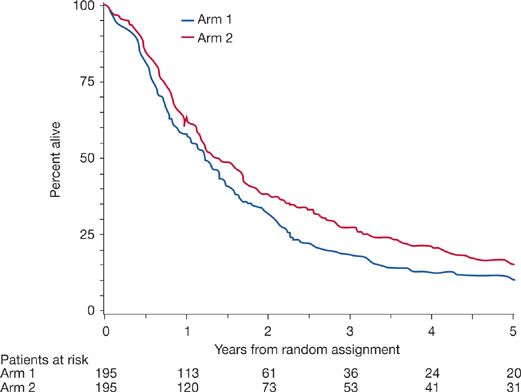
There is now strong evidence that concurrent chemoradiotherapy is better than sequential induction chemotherapy followed by radiotherapy. Several randomized trials have addressed this topic, and most show a clear benefit in local–regional control, median survival, and 2- and 3-year survival in favor of concurrent therapy (Fig. 115-1).13–15 A meta-analysis showed significant overall survival benefit with an absolute benefit of 5.7% at 3 years and 4.5% at 5 years. The concomitant treatment also reduced locoregional progression significantly but there was no difference in distant progression between the sequential or concurrent treatment.16 Long-term toxicity rates appear similar between sequential versus concurrent chemoradiotherapy; however acute toxicity with esophagitis is markedly increased with concurrent therapy without any increase in acute pulmonary toxicity.16
Figure 115-1 Five-year survival results for patients assigned to recrive standard radiation with concurrent chemotherapy compared with patients assigned to receive sequential chemotherapy and radiotherapy. Hazard ratio for death = 0.812, 95% confidence interval = 0.663 to 0.996, P =.046, two-sided log-rank test. Total dead at any time: Arm 1 = 189 and Arm 2 = 185. Slash marks indicate censored observations. (Reproduced with permission from Curran WJ Jr1, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 941. J Natl Cancer Inst. 2011;103(19):1452–1460.)
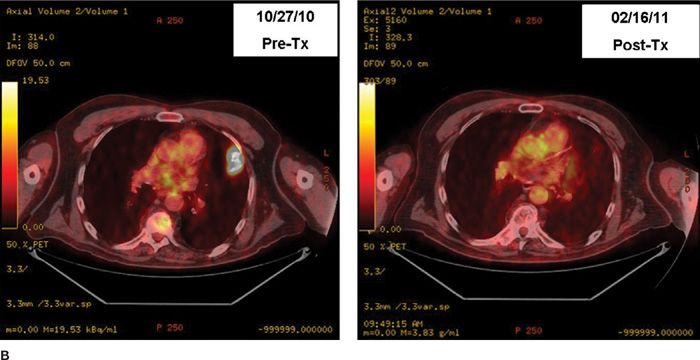
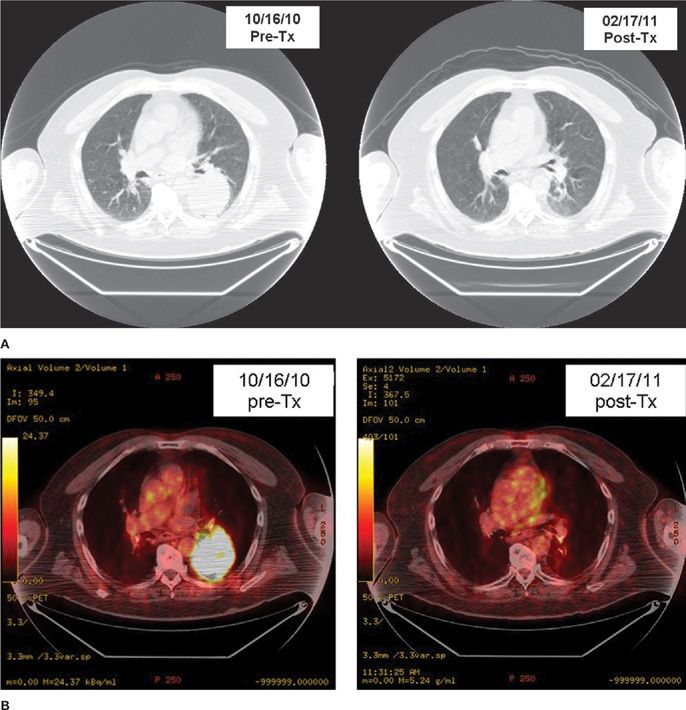
The conventional dose fractionation schedule used for definitive irradiation is 60 to 66 Gy in standard fractionation (1.8–2 Gy once daily). The maximum tolerated dose of irradiation is probably higher than that for small- to medium-sized tumors in which the amount of normal tissue in the field is low. However, preliminary results from a randomized trial did not show a benefit to 74 Gy as compared with 60 Gy.17 The standard chemotherapy drugs are a platinum-based doublet regimen. Figures 115-2 and 115-3 show examples of radiographic response to combined chemoradiotherapy in two patients with NSCLC.
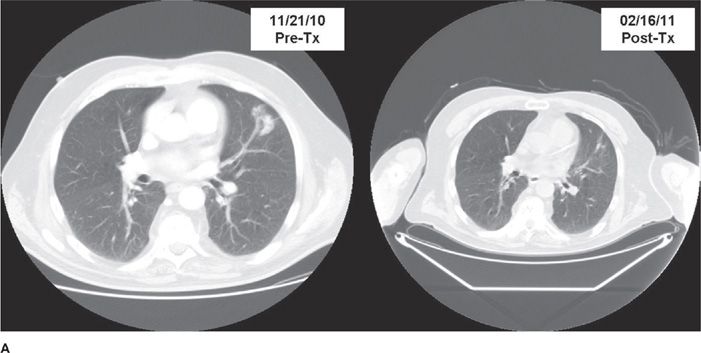
Figure 115-2 A. Pre- and postchemoradiotherapy CT scan of a patient with T1N2M0 non–small-cell lung carcinoma of the left upper lobe lung region. B. Pre- and postchemoradiotherapy PET scan of the patient in A.
Figure 115-3 A. Pre- and postchemoradiotherapy CT scan of a patient with medically inoperable T3N1 non–small-cell lung carcinoma in left lower lobe lung. B. Pre- and postchemoradiotherapy PET scan of the same patient shown in A. (Data from multiple prospective trials of the Radiation Therapy Oncology Group (RTOG) and other clinical trials.)
For many years, the fields to be treated in the definitive therapy of NSCLC have followed the Halsted principle of “radical en bloc” locoregional therapy (Fig. 115-4). The typical radiotherapy field encompassed the primary tumor (with approximately 2 cm margin), both the ipsilateral and contralateral hila, and the entire mediastinum from the thoracic inlet to a point at least 5 cm below the carina. Elective supraclavicular nodal irradiation was also typically used for upper lobe cancers. This usually results in a field size measuring approximately 16 ×20 cm, which incidentally irradiates a large amount of normal tissue. With this technique, it has been estimated that greater than 30% of a patient’s normal lung tissue is exposed to a dose of irradiation expected to cause permanent fibrosis.
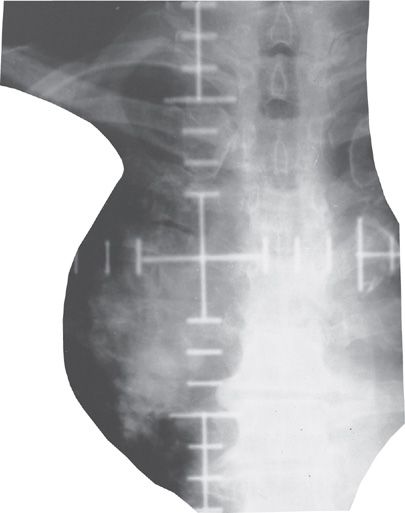
Figure 115-4 Radiotherapy simulation film (highly magnified) of a patient with medically inoperable NSCLC. The area being irradiated is showing here with the actual radiation field. CT-assisted radiation dosimetry revealed the amount of normal lung tissue in the treated field to be under 15%.
Because of these issues, in recent years there has been a trend toward smaller field size in definitive radiotherapy, encompassing gross disease with an appropriate margin and fewer areas of “prophylactic” nodal stations (Fig. 115-5). This evolution has been accelerated by improvements in preradiotherapy imaging (e.g., PET scan–based treatment planning) and the addition of chemotherapy to control microscopic disease).18 Several studies have confirmed nodal failure to be 7% to 10% if prophylactic nodal irradiation has been omitted.19,20 The use of smaller field sizes makes radiotherapy better tolerated and offers the possibility for higher doses of radiotherapy in combination with chemotherapy. A concern about local failure just outside of the irradiated volume (also known as “marginal miss”) with these newer techniques exists; however, the risk of this kind of failure appears to be relatively low compared with central local or distant failure. Most studies show that the risk for a marginal miss recurrence is between 5% and 10%, compared with 30% to 60% risk for central local recurrence and/or distant metastases. A randomized trial that enrolled 200 patients with inoperable stage III NSCLC showed similar 5-year survival rates with “involved field” irradiation versus elective nodal/comprehensive irradiation. Notably, overall response rates and local control rates were better, and toxicity lower, in association with use of the smaller fields.21
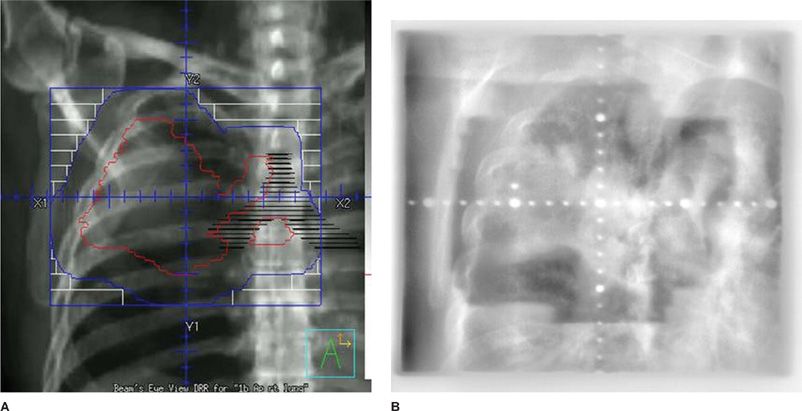
Figure 115-5 A. Digitally reconstructed radiograph (DRR) (radiation planning) film for “radical en bloc” radiotherapy for a patient with T4N2M0 non–small-cell lung carcinoma of the right upper lung. The actual area being irradiated is inside the blue boundaries. All other areas are shielded via primary collimation or secondary multileaf collimation. B. Portal imaging of the same field in (A) taken on the actual treatment machine verifying the treatment field.
Through advances in chemoradiotherapy over the past 20 years, there have been improvements in the prognosis for locally advanced, unresectable NSCLC, as reviewed in Table 115-3. With supportive care alone (e.g., antibiotics, expectorants, oxygen, etc.) expected survival is less than 6 months and only about 5% of patients are alive at 2 years. Single-agent radiotherapy improves the median survival to about 9 months, with approximately 20% of patients alive at 2 years. Sequential induction chemotherapy followed by radiotherapy further improves these values to approximately 14 months and 33%, whereas concurrent chemoradiotherapy increases these values to 17 months and 40%, respectively. Several phase II studies incorporating newer chemotherapy agents/schedules with modern radiotherapy have reported median survival of about 2 years, with approximately 20% 5-year survival. Interpretation of these improved outcomes is confounded by patient selection bias and stage migration, particularly with the widespread use of PET scan–based staging. Improvement in supportive care is also likely a contributing factor. However, the improvements in the prognosis for stage III nonoperative NSCLC are well documented by large, prospective randomized trials and should be considered valid. It must be stressed that the trials in which the outcomes were positive involved patients with good performance status, absence of malignant pleural effusions, and minimal weight loss.22 Not all patients with presumed unresectable disease would benefit from highly aggressive concurrent chemoradiotherapy.