Pulmonary metastatic disease
Kaposi’s sarcoma
Wegner’s granulomatosis
Microscopic polyarteritis
Behcet’s disease
Systemic lupus erythematosus
Idiopathic pulmonary hemosiderosis
TB
Mycetoma
Respiratory viral infections
Lung abscess
Parasitic disease
Von Willebrand’s disease
Haemophilia
Thrombocytopenia
Disseminated intravascular coagulation
Bronchitis
Cystic fibrosis
Foreign body
Iatrogenic
Pulmonary sequestration
Endometriosis/catamenial
Lymphangioleiomyomatosis
Cryptogenic or idiopathic
Pulmonary emboli
Pulmonary hypertension
Congestive cardiac failure
Mitral stenosis
History
Haemoptysis needs to be differentiated from haematemesis, epistaxis or bleeding from the oropharynx. This information is sometimes more difficult to elicit than one may think, and confirmation of understanding of what constitutes coughing blood needs to be made. An assessment of volume also needs to be performed. Doing this accurately is difficult. A small volume of blood can seem large, especially when the reporter is frightened. If the patient is in hospital, ongoing measurement of volume needs to be documented. In the era of smart phones, patients often photograph initial episodes of haemoptysis, so it is worth asking about. Onset and frequency then needs to be established. In women, take note of association with menses (catamenial haemoptysis). The patient may describe a gurgling sound, which could indicate the side from which the bleeding is coming. A clear, detailed history will help identify or exclude causes.
It is important to establish associated symptoms that may suggest a particular aetiology. For example, the presence of fevers and night sweats may suggest infection. Associated weight loss should prompt immediate exclusion of malignancy and TB. Acute breathlessness and chest pain may suggest infection or pulmonary embolism. Associated sputum production acutely may suggest pneumonia or bronchitis but chronically may suggest bronchiectasis.
A full systemic review should be performed. For example, joint pains and rashes should prompt investigation for vasculitis. A thorough past medical history needs to be made, with particular attention to childhood respiratory disease (bronchiectasis and TB reactivation). Cardiovascular disease can cause haemoptysis, so enquire about previous cardiovascular disease, paroxysmal nocturnal dyspnoea, orthopnoea and peripheral oedema.
It is important to elicit a drug history, in particular, anti-coagulation and anti-platelet therapy. Smoking and travel history may also add information to assessment of risk for lung cancer, TB and pulmonary embolism. A summary of key points of the history is made in Table 10.2.
| History of presenting compliant | |
| Establish true haemoptysis Frequency Weight loss Sputum production Rashes Chest discomfort Shortness of breath Recent travel Calves tenderness Orthopnoea Association with menses | Onset (acute vs chronic) Progression Volume Fevers Night sweats Wheeze Recent illness/Ill contacts History of trauma Peripheral oedema Paroxysmal nocturnal dyspnoea |
| Past medical history | |
| Previous TB/TB exposure Recent operations Recent bronchoscopy Previous breast colon or renal cancers HIV status | Previous lung disease History of PE/DVT Cardiovascular disease Childhood illness/failure to thrive |
| Drug history | |
| Allergies Anticoagulation/anti-platelets | |
| Social history | |
| Smoking and pack year history Alcohol intake | |
| Family history | |
| Congenital illness | |
Examination
After the history is taken, assessment of the patient’s physical status is essential (In the case of massive haemoptysis, this will be performed simultaneously.) Observations should be recorded, and an assessment of physiological compromise should be made. This should involve the application of an early warning score if in hospital.
The examination may be normal. Features on general examination to ascertain are the presence or absence of cachexia, pallor, cyanosis, clubbing, lymphadenopathy, rashes, telangiectasia or ecchymoses.
Respiratory examination is mandatory. Clinical signs of deep vein thrombosis should be excluded. In addition, a cardiovascular examination should be performed to ascertain signs of heart failure, pulmonary hypertension or valvular disease.
Abdominal examination may reveal signs of hepatic failure, congestion, intra-abdominal masses or scars.
Investigations
Most patients will have small-volume haemoptysis and can be investigated as an outpatient[16]. The National Institute for Clinical Excellence (NICE) lung cancer guidelines suggest urgent (2 week wait) referral to respiratory medicine for patients with persistent haemoptysis who are smokers or ex-smokers aged 40 years and older. They suggest an urgent chest radiograph for all patients with haemoptysis to be arranged by their general practitioner (GP)[17]. Investigations will be guided by history and examination.
Chest radiograph is an essential test, as it will rapidly identify gross pathology such as tumours and consolidation and may help localize the site of bleeding. It will fail to identify pathology in up to 46% of cases[18].
Initial blood tests should include full blood count, urea and electrolytes, liver function and clotting. Second-line tests that may offer a diagnosis are anti-neutrophil cytoplasmic antibodies or anti-nuclear antibodies if concerned about a vasculitis or autoimmune process. If vasculitis is a possibility urine analysis (protein and blood) and microscopy (for cast cells) should also be performed.
Sputum examination and culture are important to exclude bacterial infection and tuberculosis[19].
Computed tomographic (CT) scanning increases diagnostic yield, especially in distal bronchial and parenchymal abnormalities. It should be done prior to bronchoscopy as it will help locate the abnormality, improving diagnostic yield especially in cases of possible malignancy[17,20]. The choice of scan and protocol will depend on the likely underlying cause – for example, CT staging in cases of malignancy and high-resolution CT scan in cases where it is felt to be due to an interstitial process or bronchiectasis. CT may fail to identify small endobronchial lesions, early mucosal abnormalities, bronchitis, squamous metaplasia and a benign papilloma[21]. CT angiography may also be performed to assess bronchial arteries and potential bleeding source. This should be performed in patients with known bronchiectasis or cystic fibrosis when bronchial artery embolization is the treatment of choice if conservative options fail.
Fibreoptic bronchoscopy should be considered in high-risk patients if the chest X-ray and CT scan are normal and no cause has been found for the haemoptysis such as infection. Those at high risk include ongoing haemoptysis, smokers and those over the age of 40[22]. This could be diagnostic, allowing visualization of the airways and endobronchial or transbronchial biopsies to be taken. This could also be therapeutic; interventions such as cold saline, adrenaline or balloon tamponade can be performed[23].
Second-line investigations include CT pulmonary angiogram (CTPA) to rule out pulmonary embolism, echocardiography to assess for pulmonary hypertension and nasopharyngoscopy performed by an ear, nose and throat specialist may be beneficial.
Management of haemoptysis
The sequence and timing of investigations and management will depend on whether the patient is having a massive or non-massive haemoptysis. The next section describes an algorithm for investigation and management. This is summarized in Figure 10.1.
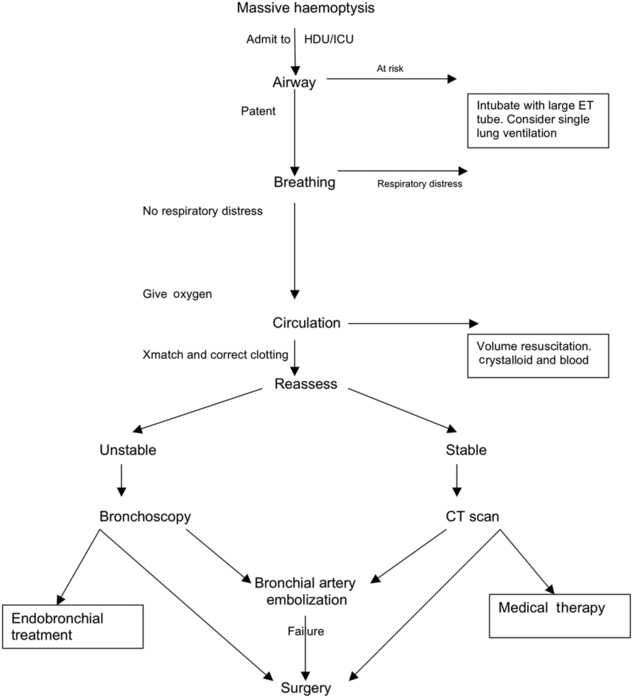
Management of massive hemoptysis.
The mortality rate for patients with mild to moderate haemoptysis is low (2.5 and 6%, respectively)[13]. Investigations and management are similar to that of massive haemoptysis; however, conservative approaches play more of a role and surgical intervention less.
Management of massive haemoptysis
Patients should be resuscitated in accordance with the Resuscitation Council (UK) guidelines, using the ABCDE approach in assessment and management[24]. Senior doctors from intensive care and respiratory medicine should be called immediately. These patients are best managed in a high dependency or intensive care setting and nursed bleeding side down (if known)[19]. Thoracic surgery and interventional radiology need to be informed at an early stage.
The immediate priority, to prevent asphyxiation, is to protect the airway and ensure adequate oxygenation. This may require tracheal intubation, in which case a large-caliber single-lumen ET tube should be inserted. It should be large enough to allow suctioning and the introduction of a fibreoptic bronchoscope, preferably size 8 or more[25]. Once the airway is clear, if the side of haemoptysis is known, unilateral intubation can be performed, allowing protective ventilation of the collateral lung and preventing aspiration from the side actively bleeding. This can be done with a double-lumen tube; however, this requires equipment and expertise to prevent misplacement and the complications that may arise as a result[26]. An alternative to protect the left lung is, under fibreoptic bronchoscopic guidance, the left main bronchus is intubated. This is best avoided on the right, as a pitfall is the inadvertent occlusion of the right upper lobe, preventing it from participating in gaseous exchange. In this situation, an ET tube is inserted over a bronchoscope; and a Fogarty catheter of size 14F/100 cm length is passed through the vocal cords and guided via bronchoscopy in to the left main bronchus and inflated, allowing continued ventilation of the right lung and tamponade of the left[19].
Two large-bore cannulae should be inserted to allow adequate volume replacement. Samples should be sent for full blood count, urea and electrolytes, liver function tests, cross-match, clotting and inflammatory markers. Cross-match at least 6 units of blood. Coagulopathy should be corrected, which may include reversing anticoagulants[7]. Arterial blood gases are useful to look at parameters such as lactate, pH and base excess and provide an instant estimate of haemoglobin concentration. Replace volume with crystalloids and blood products, once available. Tranexamic acid can be given; however, there is little evidence to support its use. Other medical treatments that should be commenced include antibiotics for infection, anti-tuberculous therapy in the case of TB and systemic antifungals for Aspergillus lung disease.
Localizing the bleeding
Once the patient has been stabilized, the next priority is to locate the bleeding point. The patient may need transfer to a specialist centre if he or she is being managed in a unit without interventional radiology or thoracic surgery. If that is the case, an expert in transfer medicine should assess the patient.
There is no consensus on the best diagnostic approach to massive haemoptysis. Broadly, though, an assessment of stability needs to be made. In the case of haemodynamic instability (where time taken for CT scanning could threaten life), patients should proceed immediately to bronchoscopy. Exceptions to this are patients with known bronchiectasis or cystic fibrosis. These patients, no matter how severe the bleeding is, should not undergo bronchoscopy at this stage.
Bronchoscopy
Urgent rigid bronchoscopy should be preformed where possible; it is preferable to fibreoptic bronchoscopy for maintaining airway patency, preserving ventilation, allowing for suctioning and therefore better visualization of the airways. Definitive procedures can also be performed. The major limitation of rigid bronchoscopy is the inability to identify more peripheral lesions or view the upper lobes easily; however, a fiberoptic bronchoscope can be introduced through it. It is more suitable for patients with large-volume active bleeding requiring endobronchial management or in cases with bilateral lung involvement, where radiographic intervention may be limited[23]. Bronchoscopy identifies the site of bleeding in 73–93% of episodes of massive bleeding[27,28].
Therapeutic techniques include instillation of epinephrine (1:20,000) to a bleeding point; however, its effectiveness is uncertain in life-threatening haemoptysis[25]. There is limited evidence for the use of cold saline lavage, fibrinogen compounds and tranexamic acid[29–31]. Endobronchial balloon tamponade can be performed. This involves identifying the site of bleeding and wedging the bronchoscopy against it. A 4–7F Fogarty catheter is inserted through the bronchoscope and inflated. The bronchoscope can then be removed over the catheter, which is left in place for 24 hours. Complications with balloon tamponade include mucosal necrosis and obstructive pneumonia[19].
Neodymium: yttrium-aluminium-garnet (Nd:YAG) laser photocoagulation has been used with some success in the literature[32].
Stable patients
Haemodynamically stable patients with no evidence of respiratory distress should have a CT scan. CT is more effective than bronchoscopy at locating the site of bleeding and identifying the aetiology of the bleeding (60–77 vs 2.5–8%)[23]. New multi-detector CT scans allow more accurate identification of bronchial and non-bronchial systemic arteries in comparison with conventional angiography; quoted success rates are 62–100%[33]. This can be used for planning for bronchial artery embolization.
Bronchial artery embolization (BAE)
BAE (Figure 10.2) is a minimally invasive procedure that is well established in the management of massive haemoptysis. Success depends on the underlying disease, with better outcomes in TB and less favorable outcomes in lung cancer. Immediate control of haemoptysis is achieved in 73–99%, but the overall recurrence rate long term is unchanged[34].
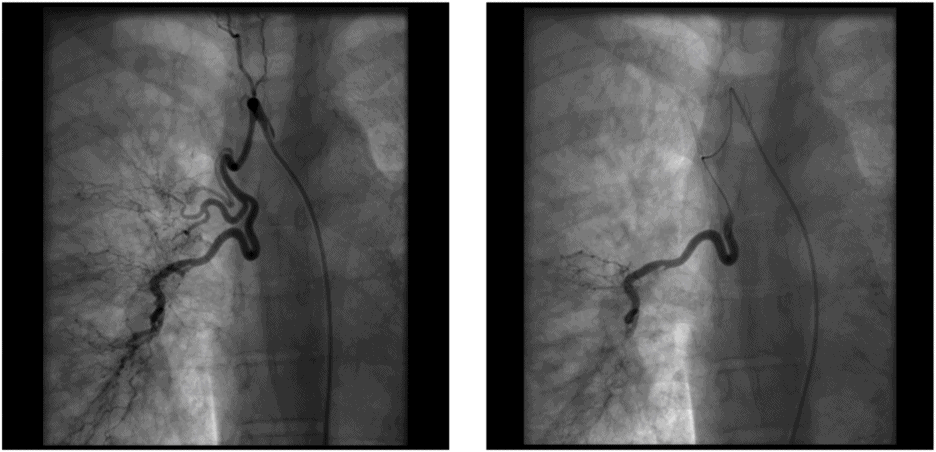
Images demonstrate bronchial arteries pre- and postembolization
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


