Fig. 19.1
Time to treatment monotony. Collation of selected clinical trials over the past two decades arranged according to time from symptom onset to commencement of reperfusion therapy. Note that for FINESSE and ASSENT 4 PCI, the solid component of the bar represents fibrinolytic therapy whereas the hatched area represents time to commencement of primary PCI. Time to primary PCI only is represented for APEX-AMI
A profile of patients who exhibit delays in presentation after symptom onset has emerged, indicating that those who are elderly, female, diabetic, African American, or of lower socio-economic status are most likely to delay calling for help [10, 11]. Results from a recent clinical trial emphasize the growing importance of this aspect in the elderly: hence among nearly 6,000 STEMI patients undergoing primary PCI randomized within 6 h of symptom onset, 17 % were over 65 years, yet this group accounted for 64 % of the deaths [12]. An additional public health challenge relates to the choice of transportation to a health care facility given that at least 50 % of patients do not employ the emergency medical services (EMS) system, but rather present as “walk-ins” to the emergency room [13]. Patients who self-present in this way are subject to further delays in diagnostic recognition and therapy. The ongoing initiative of the American Heart Association and the National Heart, Lung, and Blood Institute (NHLBI) “act in time to heart attack signs” (http://www.nhlbi.nih.gov/health/public/heart/other/amer-indian_risk/sec2/) highlights the heart attack warning signs, encourages calling 911 promptly, and articulates an appropriate planning process for patients and their families. In Fig. 19.2, a collage of three data sets underscores the relationship between elapsed time from coronary occlusion (or symptom onset) and myocardial salvage, lives saved, and the frequency of aborted myocardial infarction with reperfusion therapy. As shown, in the original canine experiments of Jennings and Reimer, ischemic necrosis begins in the sub-endocardium within 20 min of coronary occlusion and proceeds in a transmural wavefront of cell death that maximizes within 3–6 h [15]. Reperfusion within the first hour salvaged almost two-thirds of the myocardium at risk, but thereafter salvage abruptly declined. The original placebo-controlled trials of fibrinolysis also highlight that maximum benefit on 35-day mortality was achieved when treatment was received with 60 min of symptom onset [16]. This is well aligned with the avoidance of myocardial necrosis and the concept of aborted myocardial infarction, which mirrors both the experimental canine evidence and the early fibrinolytic trials [17]. Hence one in four patients treated within the first hour of symptom onset exhibits complete resolution of their initial ST elevation with minimal or no myocardial necrosis [18]. Although the slope of efficacy over time appears somewhat shallower for PCI than fibrinolysis, using cardiovascular magnetic resonance data, Francone et al. examined different total ischemic time intervals to primary PCI and found that myocardial salvage markedly decreased when symptom onset to balloon time exceeded 90 min [10]. As evident in Fig. 19.3, not only did mean infarct size progressively increase after this time point, but the likelihood of microvascular obstruction was also greater in patients reperfused later [19].
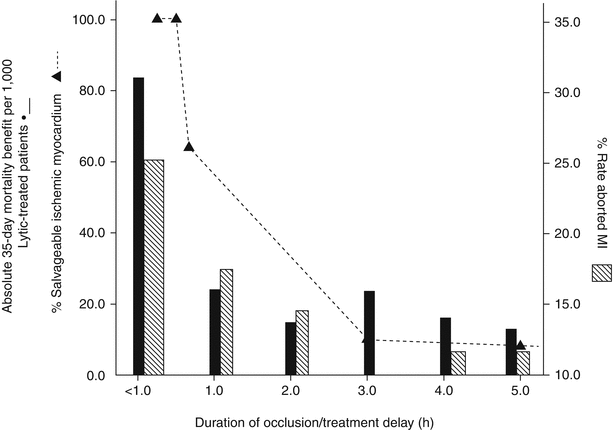
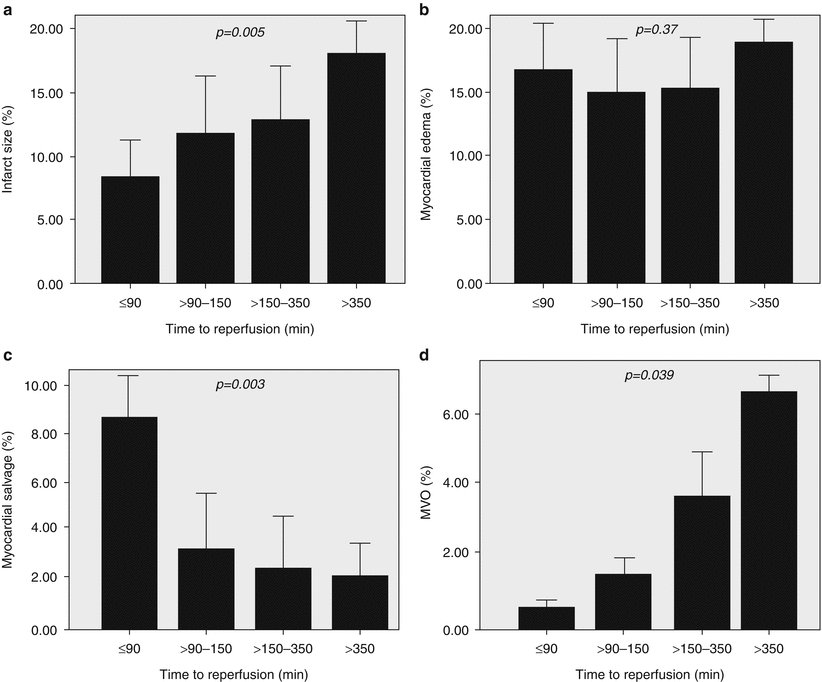

Fig. 19.2
Relationship between elapsed time and myocardial salvage, lives saved, and frequency of aborted myocardial infarction (MI) with reperfusion therapy. Dashed line represents percent of canine left ventricle salvageable after coronary occlusion/reperfusion. Solid bars represent number of lives saved per 1,000 patients treated with fibrinolysis according to time from symptom onset. Hatched bars represent proportion of aborted myocardial infarction in fibrinolytic-treated patients according to time from symptom onset (From Armstrong et al. [14]. Reprinted with permission from Wolters Kluwer Health)

Fig. 19.3
Cardiovascular Magnetic Resonance (CMR) data from 70 ST-elevation myocardial infarction patients successfully treated with primary PCI. Data in the four panels are expressed according to time from symptom onset to reperfusion and stratified according to quartiles for: infarct size (a), myocardial edema (b), myocardial salvage (c), and microvascular obstruction (MVO) (d). Note infarct size rises and salvage declines after time from symptom onset exceeds 90 min. Note: MVO also rises after this point (From Francone et al. [19]. Reprinted with permission from Elsevier Limited)
Since the last edition of this text [1], an important initiative in the care of STEMI patients has developed to evaluate quality improvement activities promulgated by the ACC and AHA. Specifically, the American College of Cardiology’s National Cardiovascular Data Registry (NCDR) has engendered an Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get With The Guidelines (AR-G) that evaluates both patient and hospital characteristics as well as rates of guideline adherence, procedural details, and in hospital outcomes in STEMI. The initial report, encompassing a 2.5 year period ending in the second quarter of 2009, comprised over 20,000 STEMI patients from approximately 250 participating centers [20]. Key findings during this period of observation were greater use of reperfusion in eligible STEMI patients; increased use of PCI and a decline in fibrinolytics; reduced door to balloon times in primary PCI centers such that the large majority of such patients were achieving this with 90 min; and a reduction in procedural vascular complications and an increase in the application of secondary preventative measures and evidenced–based medicines. The most recently available data from this registry are shown in Fig. 19.4. While the achievement of consistent door to balloon times of approximately 1 h for patients not requiring transfer to PCI centers is encouraging, the persisting delay in achieving timely PCI in those patients transferred from other institutions (representing the majority of STEMI patients in the U.S.) is discouragingly high (i.e., approximately 2 h). Importantly these times do not take into account total ischemic time; therefore, the time from symptom onset to first medical contact, as well as their baseline risk, is unknown.
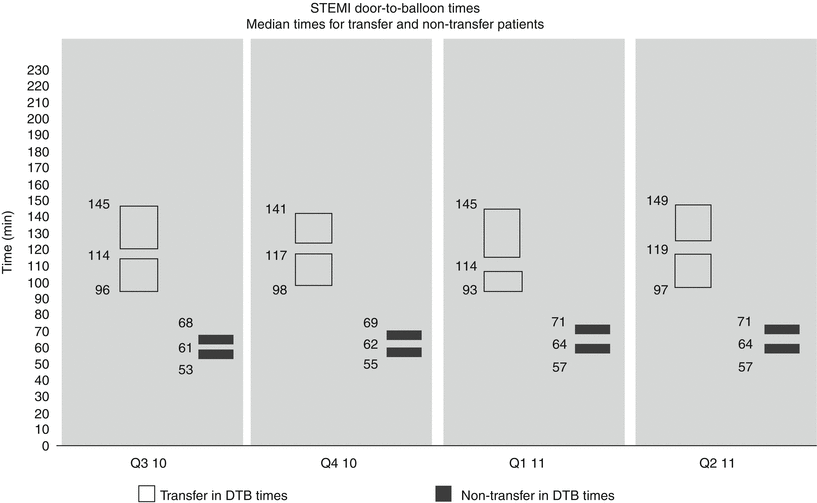

Fig. 19.4
Temporal trends in door (first door) to balloon times for STEMI patients undergoing primary PCI in the ACTION Registry by quartile for the year ending June 30, 2011. Times are medians with interquartile ranges for those (solid bars) presenting to PCI centers versus those presenting to non-PCI centers requiring transfer for PCI (open bars) (Reproduced with permission from the ACTION Registry-GWTG National slide set Q3 2010-Q2 2011)
Since baseline risk strongly intersects with time from symptom onset to reperfusion in modulating reperfusion strategies, an appreciation for the wide spectrum of risk is critical. As demonstrated by Morrow et al. [21], the large majority of STEMI patients in the substantially populated NRMI-3 registry are at low risk (i.e., TIMI risk score <5) (Fig. 19.5). The consequence of these observations, which are too often overlooked, is that the major source of mortality arises from those at high risk (i.e., the approximate 1 in 4 patients who have a TIMI risk score ≥5): hence, it is these patients who especially benefit from timely PCI. Further insight into the anticipated margin of benefit among specific subgroups according to the assessment of baseline risk is also feasible from the Fibrinolytic Trialists’ overview analysis [16].
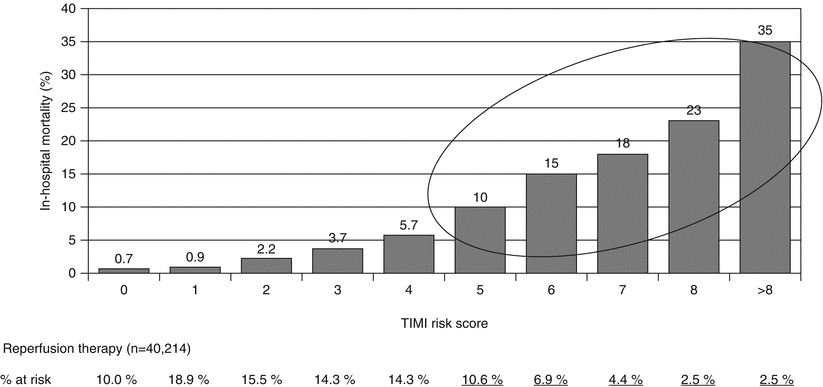

Fig. 19.5
NRMI 3- Prediction of in-hospital mortality of reperfused patients (38 % received primary PCI, 62 % fibrinolysis) according to TIMI risk score for STEMI. Patients with TIMI risk score greater than or equal to 5 account for 26.9 % of the overall population. STEMI indicates ST-elevation myocardial infarction; NRMI indicates National Registry of Myocardial Infarction (From Morrow et al. [21]. Adapted with permission from American Medical Association)
Although clear improvement in shortening door to balloon times for patients presenting de novo to primary PCI has occurred, it appears far more challenging to meet this target in the majority of hospitals in the U.S. that are community based [20]. Hence, less than one in four of such patients achieve a 90-min target and are thus deprived of the potential benefits of prompt pharmacologic reperfusion. Accordingly, a more integrated approach using both fibrinolysis and primary PCI coupled in the so-called pharmacoinvasive approach in selected locales has demonstrated excellent clinical outcomes in consecutive observational series [7, 22]. We believe that an integrated therapeutic strategy may serve the majority of patients best, taking into account the four factors originally highlighted in the ACC/AHA guidelines: (1) risk of the index event, (2) the risk of fibrinolysis, (3) time from both symptom onset, and (4) the expected time to reliably acquire expert PCI. Integrating these four factors and others as illustrated in Fig. 19.6 on a system-wide basis is likely to serve the broad cross-section of patients best [23].
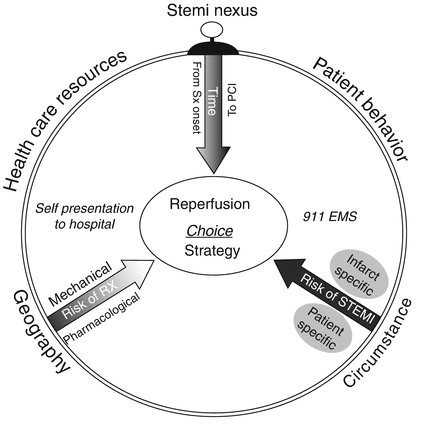

Fig. 19.6
Conceptual model of ST-elevation myocardial infarction nexus modulating choice of reperfusion strategy. The factors that compose the external environmental framework surround the model. Risks related to patient, infarct, and reperfusion-specific factors converge with both time from symptom onset and the expected time to achieve PCI. Sx indicates symptoms, EMS emergency medical service, PCI percutaneous coronary intervention, Rx treatment, STEMI ST-elevation myocardial infarction (From Armstrong et al. [14]. Reprinted with permission from Wolters Kluwer Health)
The transformative 2004 ACC/AHA STEMI guidelines have had a major impact on the development of systems of STEMI care [23]. The paradigm developed therein for emergency medicine response depicted in Fig. 19.7 suggests that EMS dispatch should occur within 1 min of a 911 call and on scene arrival achieved within 8 min of dispatch. Performance of 12-lead ECGs and consideration of prehospital fibrinolysis, if appropriate personnel and oversight are positioned to deliver it promptly, are part of the paradigm. The figure and accompanying legend outline the spectrum of options for initial transportation of STEMI patients and reperfusion strategies.
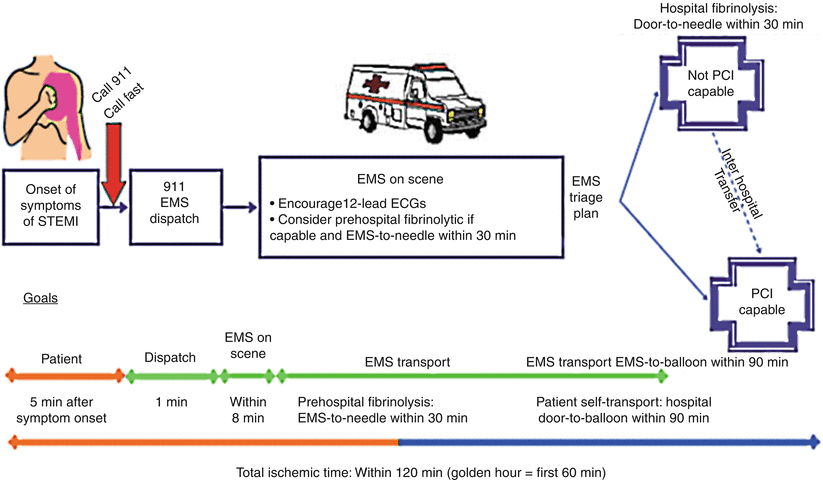

Fig. 19.7
Options for transportation of STEMI patients and initial reperfusion treatment. Patient transported by emergency medical services (EMS) after calling 911. Implementation of these reperfusion strategies will vary based on patient mode of transportation and receiving hospital capabilities. Transport time to the hospital is variable from case to case, but the goal is to keep total ischemic time within 120 min. There are three possibilities: (1) If EMS has fibrinolytic capability and the patient qualifies for therapy, prehospital fibrinolysis should be started within 30 min of EMS arrival on the scene. (2) If EMS is not capable of administering prehospital fibrinolysis and the patient is transported to a non–PCI-capable hospital, the hospital door-to-needle time should be within 30 min for patients in whom fibrinolysis is indicated. (3) If EMS is not capable of administering prehospital fibrinolysis and the patient is transported to a PCI-capable hospital, the hospital door-to-balloon time should be within 90 min. Interhospital transfer: It is also appropriate to consider emergency interhospital transfer of the patient to a PCI-capable hospital for mechanical revascularization if (1) there is a contraindication to fibrinolysis; (2) PCI can be initiated promptly (within 90 min after the patient presented to the initial receiving hospital or within 60 min compared to when fibrinolysis with a fibrin-specific agent could be initiated at the initial receiving hospital); (3) fibrinolysis is administered and is unsuccessful (i.e., “rescue PCI”). Secondary nonemergency interhospital transfer can be considered for recurrent ischemia. Patient self-transport: Patient self-transportation is discouraged. If the patient arrives at a non–PCI-capable hospital, the door-to-needle time should be within 30 min. If the patient arrives at a PCI-capable hospital, the door-to-balloon time should be within 90 min. The treatment options and time recommendations after first hospital arrival are the same (Adapted from Antman et al. [23]. With permission from Elsevier. Available at http://assets.cardiosource.com/STEMI_2004.pdf. Accessed July 30, 2012)
In the 2009 focused update of the ACC/AHA STEMI Guidelines, a useful paradigm that is complementary to the aforementioned figure has been developed incorporating baseline risk and whether or not patients are initially seen at a PCI-capable facility (Fig. 19.8) [3].
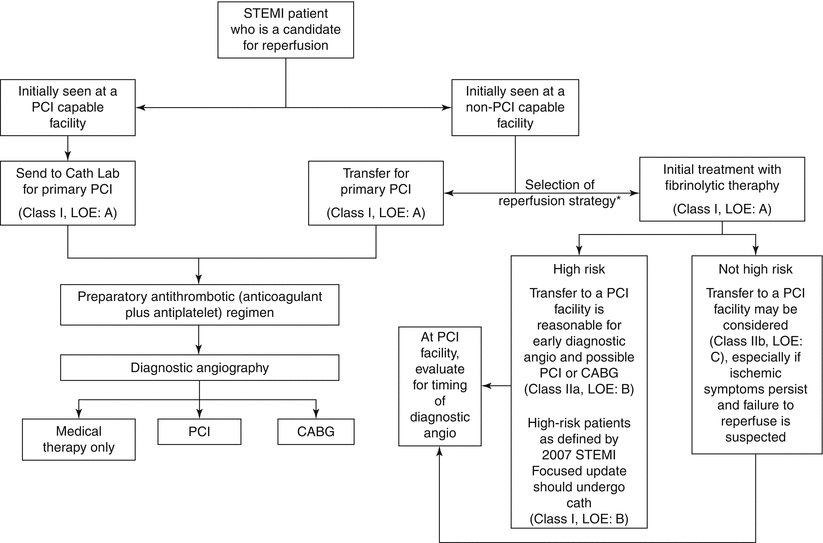

Fig. 19.8
Triage and Transfer for PCI. Each community and facility should have an agreed upon plan for treatment of STEMI. This includes which hospitals should receive STEMI patients from EMS units capable of obtaining diagnostic ECGs, management at the initial receiving hospital, and written criteria/agreements for expeditious patient transfer from non–PCI-to PCI-capable facilities. Consideration should be given to initiating a preparatory pharmacological regimen as soon as possible. Angio indicates angiography, CABG coronary artery bypass graft, Cath Lab catheterization laboratory, ECGs electrocardiograms, EMS emergency medical services, LOE level of evidence, PCI percutaneous coronary intervention, STEMI ST-elevation myocardial infarction. *Time since onset of symptoms; risk of STEMI; risks associated with fibrinolytic therapy; time required for transport to a skilled PCI laboratory (From Kushner et al. [3]. Reprinted with permission from Elsevier Limited)
The most recent 2013 ACC/AHA STEMI Guidelines emphasize advances in reperfusion therapy, organization of regional systems of care, transfer algorithms, evidence-based antithrombotic and medical therapies, and secondary prevention strategies to optimize patient-centered care [4].
Because at least one-third of the deaths from MI occur before hospital presentation, out-of-hospital cardiac arrest remains a major public health challenge. The “chain of survival” program introduced by the AHA emphasizes early recognition and bystander activation of EMS as well as prompt bystander cardiopulmonary resuscitation (CPR) and defibrillation prior to the provision of advanced cardiac life support. Cardiopulmonary resuscitation training of family members of patients at high risk and ready accessibility to automated external defibrillators (AEDs) have been shown to enhance clinically meaningful survival [23].
Choice of Therapy: Pharmacologic Reperfusion
No intervention has had a greater impact on the global management of acute STEMI than fibrinolytic therapy. The objectives of reperfusion therapy are (1) the achievement of rapid, high-quality coronary flow; (2) the maintenance of high-quality coronary patency so as to prevent recurrent ischemia and reinfarction; and (3) the enhancement of patient survival and quality of life [24]. The three core components of pharmacologic reperfusion consist of a fibrinolytic agent, concomitant antithrombotic, and antiplatelet conjunctive agents. A variety of factors influence the success of fibrinolytic therapy, including the depth, complexity, and contents of the ruptured plaque; the age of the coronary thrombus; the role of distal microembolization and small vessel occlusion; coexistent vasospasm; and endothelial dysfunction [24].
A convenient classification of currently available fibrinolytic agents begins with streptokinase, the most venerable fibrinolytic agent employed as a short-term infusion (30–60 min) in doses of 1.5 × 106 U (Table 19.1). Tissue plasminogen activator (tPA) until recently was the fibrin specific prototype and has increasingly given way to tPA congeners, tenecteplase (TNK-tPA), and reteplase (rPA). These latter agents are characterized by longer plasma half-lives than tPA, thereby permitting bolus injection that not only simplifies the administration of these agents but also reduces the potential for medication errors. Although they do not provide additional mortality reduction over that provided by front-loaded, accelerated tPA, the increased fibrin specificity afforded by TNK-tPA does confer a significant decrease in major systemic bleeding [24].
Table 19.1
Pharmacology and pharmacokinetics of fibrinolytic agents for treatment of acute myocardial infarction
Property | Streptokinase | Alteplase | Reteplase | TNK-tPA |
|---|---|---|---|---|
Molecular weight (kd) | 70 | 39 | 70 | |
Dose | 1.5 × 106 U | 100 mg/90 min | 2 × 10 IU bolus 30-min apart | 0.5 mg/kg bolus |
Plasma t 1/2α (min) | 20 | 4−8 | 15 | 20 |
Fibrin-specificity | − | ++ | + | +++ |
Antigenicity | + | − | − | − |
90-min patency | ++ | +++ | ++++ | +++(+?) |
Mortality reduction | + | ++ | ++ | ++ |
Hemorrhagic stroke | + | ++ | ++ | ++ |
Clinical development | Approved for general use | Established standard | Approved for general use | Approved for general use |
In Table 19.1, the pharmacology and pharmacokinetics of the four commercially available fibrinolytic agents are summarized. As systemic fibrinolytic therapy is administered, so too (and somewhat paradoxically) procoagulant counter-balancing forces emerge. These relate, in part, to fibrinolytic-induced exposure of surface-bound thrombin, as well as activation of platelets, which, on their surface, provide a rich source of factor Xa. A host of procoagulant factors contained in the alpha granules of platelets is also engaged. These include plasminogen activator inhibitor-1, α2-antiplasmin, platelet factor IV, and vasoconstrictor substances, such as serotonin and thromboxane A2. Table 19.2 provides an overview of the contraindications and cautions in using fibrinolytic therapy.
Table 19.2
Contraindication and cautions for fibrinolysis use in STEMIa
Absolute contraindications |
Any prior intracranial hemorrhage (ICH) |
Known structural cerebral vascular lesion (e.g., arteriovenous malformation) |
Known malignant intracranial neoplasm (primary or metastatic) |
Ischemic stroke within 3 months EXCEPT acute ischemic stroke within 3 h |
Suspected aortic dissection |
Active bleeding or bleeding diathesis (excluding menses) |
Significant closed head or facial trauma within 3 months |
Relative contraindications |
History of chronic severe poorly controlled hypertension |
Severe uncontrolled hypertension on presentation (SBP greater than 180 or DBP greater than 110 mmHg)b |
History of prior ischemic stroke greater than 3 months, dementia, or known intracranial pathology not covered in contraindications |
Traumatic or prolonged (greater than 10 min) CPR or major surgery (less than 3 weeks) |
Recent (within 2–4 weeks) internal bleeding |
Noncompressible vascular punctures |
For streptokinase/anistreplase: prior exposure (more than 5 days ago) or prior allergic reaction to these agents |
Pregnancy |
Active peptic ulcer |
Current use of anticoagulants: the higher the INR, the higher the risk of bleeding |
Assessment of Reperfusion After Fibrinolytic Therapy
Having decided to administer fibrinolytic therapy, the clinician has a cardinal responsibility to systematically assess the patient’s response over the subsequent minutes to hours. Resolution of ischemic chest pain, restoration of hemodynamic stability, and resolution of the initial ST-segment elevation by at least 50 % of its original height are helpful signs of reperfusion [23, 25]. Indeed, ST resolution is a good indicator of both macro- and micromyocardial perfusion and is aligned with better recovery of ventricular function, a lesser infarct size, and enhanced clinical outcome [25].
Emergence of an accelerated idioventricular rhythm is uncommon but has also been correlated with the achievement of infarct vessel patency. Although a variety of investigative techniques, including contrast echocardiography and magnetic resonance imaging, are under investigation, no single technique that is clinically applicable at the bedside has emerged. Hence, the clinician is left with a collage of clinical and electrocardiographic parameters: the latter can be more refined by continuous ST segment monitoring, or at least by a systematic algorithm that requires repeat 12-lead electrocardiography at 60 and 90 min [23]. Failure to achieve reperfusion may signal the need to proceed with an invasive catheter-based strategy. The Rescue Angioplasty Versus Conservative Therapy or Repeat Thrombolysis (REACT) trial investigators have demonstrated that this approach is superior to conservative therapy or repeat fibrinolysis [26, 27]. This judgment should take into account the risk of the initial infarct, other comorbidities, and the patient’s wishes.
Similarly, observation for recurrent ischemic symptoms, if associated with objective evidence of further ST segment shift or reinfarction, especially in the first 48 h after the administration of fibrinolytic therapy, is often an indication for co-intervention with catheterization and mechanical intervention.
Percutaneous Coronary Intervention
Primary PCI for STEMI will be addressed in detail in a separate chapter. The choice of the optimal reperfusion strategy for STEMI [28, 29] has been the subject of major controversy, spirited debate, and still-ongoing research [30]. It seems clear that for patients with absolute contraindications for fibrinolysis and those in Killip class III and IV, primary PCI is the preferred option, provided it can be accessed in a timely fashion by a skilled operator in an experienced facility. Appropriate triage of patients in whom primary PCI is preferred should involve a systems approach that places a strong priority on rapid EMS response, prehospital ECG, the capacity for fibrinolysis, and an enhanced state of readiness of the receiving hospital. The sensitivity to time is especially key, given the knowledge that coronary thrombus within the first 2–3 h is especially sensitive to fibrinolysis, and the Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial infarction (CAPTIM), Prague-2 and Which Early ST-Elevation Myocardial Infarction Therapy (WEST) studies demonstrate that fibrinolysis is at least as good, if not superior, to primary PCI within that select window [31–34]. It is essential that community hospitals develop or enhance effective communication strategies, with fully equipped tertiary care centers, to ensure that timely transfer of high-risk patients and those not responding to fibrinolytic therapy (approximately 30 %) can occur. Since fewer than 20 % of U.S. hospitals have facilities for primary PCI, regionalization of STEMI care has emerged as a major strategy. Regionalization of care in specialized centers has had both positive and some unintended negative effects on patient care, which are summarized in Table 19.3 [35, 36].
Table 19.3
Reperfusion paradox implications
Value added contributions | Unintended negative consequences |
|---|---|
Enhanced coordination and collaborative support of hub-spoke model | Persisting delays in accessing timely PCI in most patients presenting to non-PCI hospitals |
Greater focus on performance metrics with increased transparency across providers | Decline in ability to provide “state of the art” fibrinolytic management |
Increased emphasis on overcoming under-treatment | Proliferation of low-volume “stand-alone” PPCI centres |
Shorter times to PPCI in PCI-capable centres | Unnecessary coronary angiography or interventional procedures |
Diversion of patients from local community hospitals, with resultant potential for discontinuity of care and negative impact on long-term comprehensive secondary prevention |
Pharmacoinvasive Therapy
Based on a series of prior trials, including ASSENT 4PCI and FINESSE, the concept of using pharmacologic therapy (i.e., either full-dose fibrinolytic therapy or ½ dose with concomitant glycoprotein 2b/3a therapy followed by immediate PCI) has been abandoned [37, 38]. This relates to either diminished or no improvement in efficacy as compared with primary PCI, as well as the hazard of excess bleeding. Yet the recognized and persisting delay in achieving primary PCI in the majority of STEMI patients in clinical practice continues to generate additional clinical trials directed at achieving some initial reperfusion prior to a planned – but not immediate–mechanical coronary intervention. Encouraging data from two sources have re-fueled enthusiasm for a combined approach. The CARESS trial, which examined ½ dose fibrinolytic therapy with abciximab, and the TRANSFER trial, which studied full dose fibrinolytic therapy followed by timely PCI in ~2.8 h (the control arm also received full dose TNK and catheterization 32.5 h thereafter) support this alternative. It is noteworthy that the preferred arm of TRANSFER underwent PCI at 2.8 h as compared with the 1.6 h in ASSENT 4 PCI [39, 40]. A meta analysis of 2,961 patients randomized to early PCI after fibrinolysis versus standard fibrinolytic therapy (with variable rates of rescue PCI and invasive therapy) revealed a reduction in reinfarction and recurrent ischemia with no increase in bleeding but also no impact on mortality [41]. These data are complemented by observational data from the greater Minneapolis area in over 1,200 patients, presenting in remote areas. These investigators demonstrated that ½ dose fibrinolysis followed by transfer for PCI resulted in comparable clinical outcomes (1 year survival) to those presenting directly to a PCI center and receiving primary PCI [22]. More definitive evidence is required to support this promising approach.
We agree with the following conclusion of the ACC/AHA STEMI Writing Committee on this subject: “Given the current literature, it is not possible to say definitively that a particular reperfusion approach is superior for all patients, in all clinical settings, at all times of the day. The main point is that some type of reperfusion therapy should be selected for all appropriate patients with suspected STEMI. The appropriate and timely use of some reperfusion therapy is likely more important than the choice of therapy, given the current literature and the expanding array of options” [23]. Interestingly, this advice, although phrased somewhat differently within the ESC guidelines, is nonetheless well aligned with the aforementioned as well as our own views, i.e. “Patients vary so much from one another that individual care is paramount, and there is still an important place for clinical judgment, experience, and common sense” [42].
Concomitant Therapy
Antithrombotic Therapy
A variety of antithrombotic agents have been developed to combat the coagulation cascade and thereby sustain the benefits of fibrinolytic therapy. A summary of potential antithrombotic partners to the four generally available fibrinolytic agents is provided in Table 19.4 [51]. The reference anti-thrombotic standard remains unfractionated heparin, even though the nonspecific binding of heparin to plasma proteins contributes to substantial variation in anticoagulant effect, and the dose–response relationships make it difficult to maintain patients in an optimal therapeutic range for a sustained period. Confirmation of the safety and efficacy of this form of conjunctive therapy for both tPA and TNK supports a bolus of unfractionated heparin of 60 U/kg (maximum 4,000 U) followed by an infusion of 12 U/kg/h (maximum 1,000 U/h) with a partial thromboplastin time (PTT) target of 50–70 s during the initial 48 h [47]. Provision for down-titration of the heparin dose 3 h after its initiation is also encouraged if the PTT is greater than 70 s. The evidence supporting the use of intravenous unfractionated heparin with fibrin-specific agents is not strong, yet it has a class I recommendation, largely based on angiographic findings of improved infarct-related patency [23]. Continuation beyond 48 h should be individualized, based on risk of pulmonary and systemic embolization and other factors. Since recurrent ischemia has been noted after sudden cessation of unfractionated heparin, it is prudent to be vigilant during this time frame and consider more gradual cessation of therapy [52]. During therapy, the platelet count should be monitored daily to assess the occurrence of heparin-induced thrombocytopenia. Support for the use of intravenous unfractionated heparin with streptokinase is less certain, and there appears to be no obvious advantage over that provided by subcutaneous heparin [23].
Table 19.4
Pharmacologic partners for reperfusion therapy
Fibrinolytic [references] | Antithrombotic |
|---|---|
Streptokinase [43] | Heparin SQ 7,500–12,500 b.i.d. or |
1,500,000 units IV in 30–60 min | Heparin 5,000 IU bolus and 1,000 IU/h (PTT 60–90) or |
Bivalirudin 0.1-mg/kg bolus and 0.25 mg/kg/ha | |
Heparin 5,000 · IU bolus and 1,000 IU/h (PTT 60–90)b | |
15 mg bolus +0,75 mg/kg in 30 min (max. 50 mg) +0.5 mg/kg in 60 min (max. 35 mg) | |
Heparin 5,000 · IU bolus and 1,000 IU/h (PTT 60–90)b | |
10 mg + 10 mg double bolus (q 30 min) | |
Heparin 5,000 · U bolus and 1,000 U/h (PTT 60–90) or | |
Weight adjusted (30–50 mg) single bolus | Heparin 60-U/kg bolus (max. 4,000 U) and 12 U/kg/h (max. 1,000 U/h) targeting a PTT of 50–70b or |
Enoxaparin 30-mg bolus and 1 mg/kg SQ b.i.d. (caution for patients >75 years of age) |
Two additional antithrombotic alternatives are recommended for patients receiving fibrinolytic therapy. Both provide for easier administration, relative stability of anticoagulant effect, greater factor Xa inhibition, and avoidance of the need for laboratory monitoring. The most extensively tested of these is enoxaparin, which for patients less than 75 years of age (with serum creatinine <2.5 mg/dl in men and 2.0 mg/dl in women), is administered as an initial 30 mg i.v. bolus followed 15 min later by a subcutaneous injection of 1.0 mg/kg every 12 h [53]. For those patients ≥75 years, the initial i.v. bolus is avoided, and the subcutaneous dose is reduced to 0.75 mg/kg every 12 h. Maintenance dosing is recommended for the duration of index hospitalization (up to 8 days), and renal function monitoring is recommended: if GFR falls below 30 ml/min, the subcutaneous regimen should be altered to 1.0 mg/kg every 24 h. Those treated with enoxaparin in the large Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment (ExTRACT) trial showed a significant reduction in the primary efficacy endpoint of death or non-fatal MI, and whereas there was some excess in systemic bleeding, there was no increase in intracranial hemorrhage [53]. In OASIS-6 (Organization for the Assessment of Strategies for Ischemic Syndromes) trial, fondaparinux (provided the serum creatinine is less than 3.0 mg/dl), as compared with unfractionated heparin, in STEMI patients receiving fibrinolysis administered in an initial dose of 2.5 mg i.v. and subsequently 2.5 mg subcutaneously once daily for the duration of the hospitalization (up to 88 days) has also received a Class I (Level of Evidence B) recommendation [54]. It should be noted, however, fondaparinux is not approved for patients proceeding to PCI where an increased incidence of catheter thrombosis was observed. Moreover, the majority of fibrinolytic data acquired with fondaparinux was in conjunction with streptokinase and compared with unfractionated heparin or placebo.
Antiplatelet Therapy
The International Study of Infarct Survival II (ISIS-2) defined the important role of aspirin in doses of 162–325 mg as enhancing survival, both when used as solo therapy and when added incrementally to streptokinase [55]. Chewable aspirin or buccal or oral administration of nonenteric coated aspirin is advisable to ensure rapid initial effect. Subsequently, lifelong therapy with lower dose aspirin (i.e., at least 81 mg enteric coated) is a mandatory component of long-term secondary prevention.
The recognition that myocardial perfusion mediated both by microcirculatory flow as well as epicardial perfusion is a key mediator of infarct size and clinical outcome has focused attention on the use of antiplatelet therapy that provides incremental antiplatelet efficacy over that afforded by aspirin alone [56, 57].
Data on the use of clopidogrel from the CLARITY and Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT) trials as it relates to inpatients with STEMI treated with fibrinolysis and aspirin within 12 h supported a Class 1 recommendation for clopidogrel 75 mg daily in STEMI patients, whether or not they have received fibrinolytic therapy [58, 59]. Additionally, for those <75 years, it is reasonable to administer a 300 mg loading dose of clopidogrel (Class IIa). Data have emerged from the TRITON study supporting the use (Class 1b) of prasugrel over clopidogrel in 3,534 STEMI patients undergoing primary PCI. This was based on a reduction in the primary composite endpoint of cardiovascular death, MI, and stroke (HR 0.68; 95 % CI 0.54–0.87; p = 0.0017) at 30 days [3, 60]. Most recently, convincing data about the use of ticagrelor compared with clopidogrel in 8,430 STEMI patients intended to undergo primary PCI has been acquired in the PLATO study, supporting an indication for reducing the primary composite endpoint of cardiovascular death, MI, and stroke (HR 0.85; 95 % CI, 0.74–0.97; p = 0.02) at 1 year [60, 61].
A summary of the current guideline recommended ancillary antithrombotic and antiplatelet therapy used in conjunction with either form of reperfusion therapy is shown in Fig. 19.9.
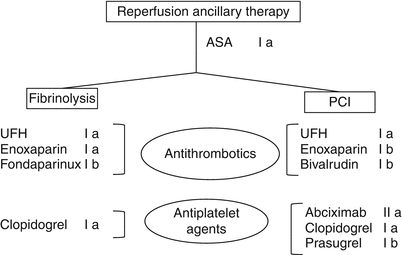

Fig. 19.9
Current guideline recommended ancillary therapy for reperfusion of STEMI (as well as ACC/AHA class of recommendation). Aspirin (ASA) is recommended for all. Note that unfractionated heparin (UHF) and enoxaparin are recommended for both fibrinolysis and PCI, whereas fondaparinux is aligned with fibrinolysis and bivalrudin for PCI. Although clopidogrel is recommended for both reperfusion strategies, the addition of abciximab and prasugrel pertains only to PCI. UHF unfractionated heparin, PCI percutaneous coronary intervention
Standard Other Initial Therapy
Supplemental oxygen therapy aimed at limiting the extent of ischemic myocardial injury remains a standard component of the therapeutic armamentarium in the initial hours after the onset of STEMI. Maintenance of an oxygen saturation of at least 90 % until clinical stability occurs, and more aggressive oxygen administration in the setting of congestive heart failure or with major hemodynamic or electrical instability is indicated. In uncomplicated STEMI cases, re-evaluation of oxygen use beyond 6 h is reasonable.
Because a component of myocardial ischemia and coronary occlusion may be mediated by coronary vasoconstriction, judicious use of sublingual nitroglycerin, employing two to three doses separated by 5–7 min, may be helpful. In instances where persisting ischemic pain, hypertension, or concomitant congestive heart failure are present, intravenous nitroglycerin beginning at an infusion rate of approximately 5–10 μg/min and titrating upward to achieve desired response is useful. When given by any route other than intravenously in acute MI, nitrates should be used cautiously, especially if hypotension, bradycardia, or right ventricular infarction is present. It is also prudent to inquire about the patient’s prior use of phosphodiesterase inhibitors for erectile dysfunction, since these agents potentiate the blood pressure-lowering effect of nitrates, and their combination could unnecessarily exacerbate the clinical course.
Although patients are encouraged to call 911 in 5 min or less from the onset of chest pain and after taking only one sublingual nitroglycerin in the ACC/AHA guidelines [23], patients with established stable angina that is severe and recurrent sometimes require two to three nitroglycerin tablets and 5–10 min is often required to alleviate their usual ischemic pain. Hence, in such a circumstance, it is our view that this advice should be tempered with room for individualized therapy.
At the first point of medical contact, nonenteric aspirin (162–325 mg) should be administered in a manner that achieves rapid absorption: chewing, sucking, or swallowing a crushed preparation.
Ancillary Early Medical Therapy
The evidence supporting the use of intravenous beta-blockers in patients with acute MI is slim and has been mainly acquired in the pre-reperfusion era and thought to merit a class IIa (level of evidence B) recommendation [62], whereas intravenous beta-blockers may be useful in the setting of hypertension, tachycardia, atrial, and ventricular arrhythmias, and in patients with ongoing ischemic pain. The COMMIT study results indicate that routine intravenous beta-blockers should be avoided, especially in patients with advanced Killip class and hypotension; in these instances, an excess of cardiogenic shock was evident [63]. Oral beta-blockade, when started early and when the patient is stable, and most especially with timolol, metoprolol, or propranolol, merits a class Ia recommendation, which is further supported by COMMIT [2]. Inhibition of the renin angiotensin aldosterone system with angiotensin-converting enzyme (ACE) inhibitors, has been extensively studied in STEMI and consistently revealed a modest but significant reduction in short-term mortality (i.e., less than 1 %) [64]. This effect, however, is greater in patients with anterior infarction, pulmonary congestion, and diminished left ventricular ejection fraction where early administration of captopril, lisinopril, or enalapril is useful [65]. Care should be taken, as is the case with beta-blockers, in commencing with a low initial dose so as to avoid hypotension. Preference should be given to the commencement of ACE inhibitors in advance of beta-blockers if relative hypotension is of concern, especially in the setting of pulmonary congestion and a large MI. If genuine intolerance to ACE inhibitors exists, angiotensin receptor blockers are an appropriate alternative. Additional benefit relating to aldosterone blockade has been demonstrated using the selected aldosterone blocker eplerenone in patients with left ventricular dysfunction and heart failure complicating MI [66]. At a mean of 16 months after therapy, begun 3–14 days after MI, eplerenone significantly reduced each of the two primary end points (death 16.7 % and cardiovascular death and hospitalization 30.0 % with placebo to 14.4 and 26.7 %, respectively, with eplerenone). Patients with elevated creatinine were excluded from this study, and special care should be used in monitoring both serum potassium and creatinine, if this therapy is employed, and most particularly with concomitant use of ACE inhibitors. Although eplerenone received a class Ia recommendation and clearly may be beneficial during the convalescence of STEMI patients, less than half of the patients entered into the EPHESUS study received reperfusion therapy, and the actual proportion with ST elevation on admission is unclear [23].
Complications
Bleeding
The recognition of the adverse short and longer-term consequences of bleeding in association with STEMI care, irrespective of the choice of reperfusion therapy, has captured much attention. Large scale registries provide added insight to that of clinical trials. Whereas the trials have highlighted the potential advantages of fondaparinux and bivalirudin, the registries underscore the challenges of translating such knowledge into clinical practice [54, 67]. In this regard, observations from the NCDR (National Cardiovascular Data Registry) ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry are sobering. Among the approximately 100,000 ACS patients studied, one-third had STEMI; in these, there was a 12 % rate of in hospital bleeding [68]. As defined by the CRUSADE bleeding risk score, there was a remarkable sixfold variation in bleeding incidence [69]. Not surprisingly perhaps, many of the baseline characteristics, comorbidities aligned with the underlying risk of STEMI co-localize with bleeding risk, including age, sex, body weight, and renal function. However, the use of multiple antithrombotic and anti-platelet agents the proclivity to switch regimens (i.e., cross over during the hospital course) and frequent dosing errors are all at play contributing to adverse consequences. A familiar yet vicious cycle often emerges whereby worsening ischemia engenders more intense antithrombotic therapy, which produces bleeding with potential adverse hemodynamic consequences, the need for transfusion (with its short and long-term adverse consequences), and finally the need for cessation of antithrombotic therapy leading to worsening ischemia. In Fig. 19.10 [70], the key intersection of a variety of factors associated with the risk and benefits of such therapies is summarized.
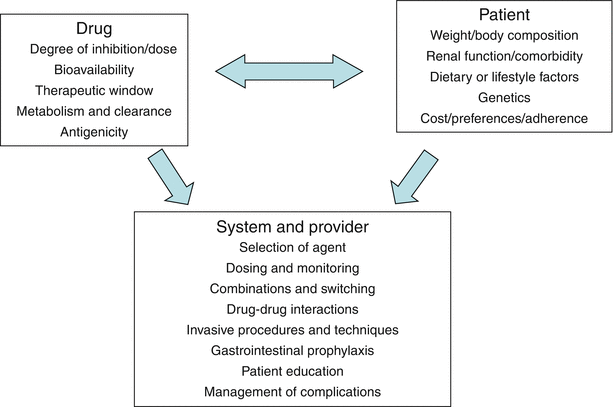

Fig. 19.10
Interdependence of factors associated with risks and benefits of antithrombotic agents. This schematic lists factors grouped as drug-specific, patient-specific, and provider-or system-specific, with arrows to reflect their interdependence (From American Heart Association: Alexander and Peterson [70]. Reprinted with permission from Wolters Kluwer Health)
Hemodynamic Complications
The development of pulmonary edema, low output, and shock are key complications in STEMI patients that require careful diagnostic evaluation in order to guide appropriate management (Fig. 19.11). A first priority requires a systematic screen for correctable causes, such as brady- or tachyarrhythmias, hypovolemia, and adverse responses to pharmacotherapy (e.g., beta-blockers or ACE inhibitors). An especially familiar context for low output in acute inferior MI is vagotonia associated with ischemic cholinergic stimulation that prompts both bradycardia and hypotension. These phenomena may be exacerbated by overzealous use of routine nitrates and intravenous diuretics. Patients with a right ventricular infarction, which occurs in approximately one third of inferior STEMI patients, present a particular therapeutic challenge [71]. Such individuals commonly respond, however, to aggressive volume loading, supplemented with intravenous dobutamine [72]. Hemodynamic monitoring with Swan-Ganz catheterization is useful in documenting right- and left-sided filling pressures and guiding appropriate therapy.
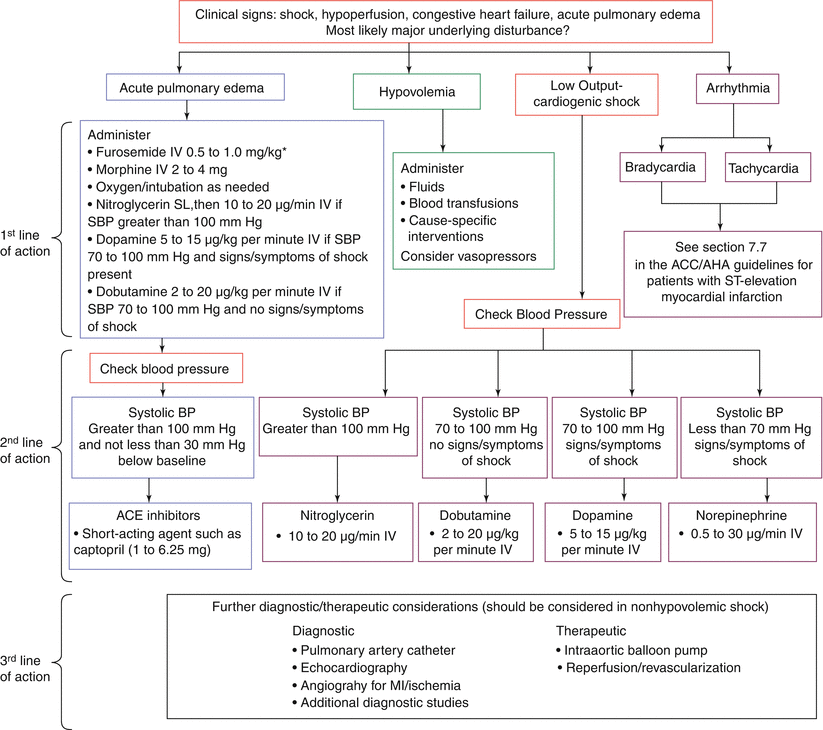

Fig. 19.11
Emergency management in complicated STEMI (From Antman et al. [23]. Reprinted with permission from Elsevier. Available at http://assets.cardiosource.com/STEMI_2004.pdf. Accessed July 30, 2012)
The appearance of a new systolic cardiac murmur in association with low output or congestive heart failure raises the specter of either a ruptured intraventricular septum or acute mitral regurgitation secondary to papillary muscle dysfunction or dehiscence. Echocardiography is especially useful in delineating evidence of a left to right shunt or mitral regurgitation, and invasive hemodynamic monitoring may also assist, not only in the diagnosis but also in guiding subsequent management. Since both of these lesions are afterload dependent, unloading the ventricle with intravenous nitroprusside, nitroglycerin, or intraaortic balloon pumping may provide the necessary bridge to stabilize such patients. However, prompt intervention with coronary angiography to define potentially correctable lesions and surgical repair in appropriate cases is required [23].
Free wall cardiac rupture with rapid hemodynamic collapse associated with electrical mechanical dissociation is a particularly devastating early complication [73]. Emergency pericardiocentesis for concomitant pericardial tamponade may, on occasion, save the patient, but surgical intervention is required. It carries a high mortality but is the only alternative. Occasionally, minor free wall perforations may actually self-seal and give rise to pseudoaneurysm formation. For STEMI patients with cardiogenic shock without demonstrable mechanical defects and who are younger than 75 years of age, compelling data from the SHOCK study support proceeding with early angiography and revascularization as appropriate [74]. Aggressive pharmacologic therapy and the insertion of an intraaortic balloon are usual preambles to angiography, and the evidence suggests that this strategy is applicable to patients who develop shock within 36 h of MI and for whom revascularization can be undertaken within 18 h of the onset of shock. Such an approach may also be appropriate for selected patients over the age of 75, recognizing that the biologic and chronologic ascertainments in patients with advanced age may differ [75]. Figure 19.11 provides a management algorithm for these hemodynamic complications of MI.
Ventricular Aneurysm
Left ventricular aneurysm formation is an important mechanical complication of MI. It occurs in association with transmural necrosis, is associated with infarct thinning and the potential for expansion, and is most common in the anterior wall when the infarct artery is occluded and there are no intercoronary collaterals [76]. Although suggested by persistent ST elevation in the setting of a Q wave, the diagnosis is best made from echocardiography or left ventricular angiography. In addition to left ventricular dysfunction and failure, aneurysms may serve as a nidus for mural thrombosis and systemic embolization as well as the substrate for major ventricular arrhythmias. Interrogation of the left ventricular apex for thrombus formation in the early days after a large anterior MI is an important investigation. Warfarin therapy is indicated in the presence of left ventricular thrombosis [23].
Recurrent Symptoms
Chest pain after STEMI is a common symptom and major focus of clinicians caring for such patients. Indeed, the frequent interrogation of patients in the early hours after presentation may provoke undue anxiety in patients, given that some residual precordial discomfort may persist for hours despite successful reperfusion. Concomitant gastrointestinal distress and anxiety may lead to the reporting of previously ignored discomfort and confound the inexperienced diagnostician. Recurrent ischemia and infarction in the first few days after presentation of STEMI portend a worsened prognosis and require careful assessment [77, 78]. Recurrent ST elevation in the distribution of the same infarct location as at presentation versus a different region (so-called ischemia at a distance) are usually indications for early angiography, unless there are obvious secondary causes or suboptimal medical therapy [79].
Recurrent ischemic pain must be differentiated from that of pericarditis, which is most common in patients with major full-thickness myocardial necrosis and extensive infarctions [80]. The more distinctive characteristics of pericardial pain, exacerbated by respiration and relieved by the upright position, as well as the physical findings of a pericardial friction rub, may be useful differential points [81]. More diffuse ST elevation with an upward concavity as well as PR depression are typical electrocardiographic findings. The appreciation of the potential for harm with nonsteroidal antiinflammatory agents and corticosteroids, based on their negative impact on healing, as well as ibuprofen’s block of the antiplatelet effect of aspirin, have modified prior treatment recommendations [82, 83]. Aspirin remains the first line of therapy, with colchicine (0.6 mg orally every 12 h) and acetaminophen as suggested alternatives. Antithrombotic therapy should be discontinued, and careful surveillance for pericardial tamponade should be undertaken clinically and as required with the aid of echocardiography.
Convalescent Care
In asymptomatic patients, the focal points of subsequent management involve thorough risk stratification, appropriate co-intervention, and the application of secondary prevention through evidence-based medication and lifestyle modification. Fundamental to the risk profile of such patients is an objective evaluation of left ventricular function undertaken by echo cardiography, nuclear cardiography, or MRI (Fig. 19.12). Patients with depressed left ventricular ejection fraction have an increased long-term morbidity and mortality and should be considered for revascularization, if appropriate. The role of electrophysiologic consultation and testing and the appropriateness and timing of device implantation are addressed in another chapter. A symptom-limited exercise test is valuable in further triaging patients without high risk features. For those in whom the ECG is uninterpretable, or physical disability precludes adequate exercise, pharmacologic stress using dobutamine, echocardiography, or adenosine/dipyridamole nuclear studies is key to detecting reversible ischemia and the desirability of proceeding with invasive study. Exercise testing may play additional useful roles in the post-STEMI patient: (1) to help evaluate the current medical pharmacotherapy, (2) to establish an exercise prescription and reassurance guide regarding functional capacity, and (3) to serve as a baseline for subsequent cardiac rehabilitation. The ability to perform at least 5 metabolic equivalents of task (METs) of exercise without early ST depression and with an appropriate rise in systolic blood pressure is a useful sign of lower risk.
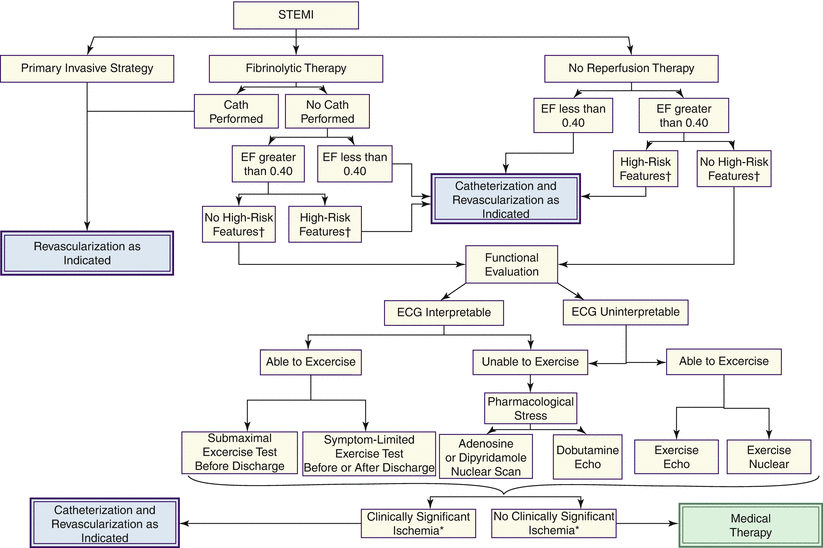

Fig. 19.12
Evidence-based approach to need for catheterization and revascularization following STEMI: algorithm for catheterization and revascularization after STEMI. The algorithm shows the treatment paths for patients who initially undergo a primary invasive strategy, receive fibrinolytic therapy, or do not undergo reperfusion therapy for STEMI. Patients who have not undergone a primary invasive strategy and have no high-risk features should undergo functional evaluation using one of the noninvasive tests shown. When clinically significant ischemia is detected, patients should undergo catheterization and revascularization as indicated; if no clinically significant ischemia is detected, medical therapy is prescribed post-STEMI. EF ejection fraction. *See the ACC/AHA Guidelines for the Management of Chronic Stable Angina (Table 23 of that Guideline) for further definition. †See Table 3, Section 6.3.1.6.2., and Section 7.3 in the full-text STEMI guideline for further discussion (From Antman et al. [23]. Reprinted with permission from Elsevier. Available at http://assets.cardiosource.com/STEMI_2004.pdf. Accessed July 30, 2012)
The controlled environment in the early days after a transforming event, such as STEMI, provides the clinician with a unique window of opportunity to engage patient and family in aggressive secondary prevention. Appropriate dietary modification to reduce weight and an exercise program developed during rehabilitation are first steps. Smoking cessation, not only for the patient but also for those who live in the same household, is especially key, and may be coupled with pharmacologic adjuncts, such as nicotine replacement therapy. Appropriate therapy for hyperglycemia, beginning acutely with an insulin infusion to achieve and maintain blood glucose levels less than 180 mg/dL while avoiding hypoglycemia and subsequently with oral hypoglycemic therapy to control hemoglobin A1C(HbA1C) to less than 7 %, is desirable [3, 23]. Intense lipid therapy with the early introduction of statins can achieve a low-density lipoprotein (LDL) cholesterol of less than or equal to 100 mg/dL. Evidence from the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) study shows that intensive lipid lowering therapy initiated within 10 days after hospital admission of acute coronary syndromes with 80 mg of atorvastatin to achieve an LDL cholesterol of less than 70 mg/dL achieved a 16 % reduction (from 26.3 to 22.4 %) in a composite end point of death, MI, unstable angina, requiring rehospitalization and coronary revascularization and stroke [84].
Daily walking should be strongly encouraged with a progressive increase in the pace and distance as tolerated. Sexual activity with the usual partner may be resumed within 2 weeks and is roughly metabolically equivalent to the ability to climb two flights of stairs. Commencement of driving after STEMI is subject to regional jurisdictions, but a waiting period of between 1 week to 1 month for private driving and 3 months for commercial driving is often recommended. However, if revascularization has been undertaken, no reversible ischemia is evident, and a good functional status demonstrated, a shorter period of time is reasonable.
Prescription of an appropriate medical program at the time of hospital discharge and re-evaluation after appropriate up-titration of medical therapy at approximately 1 month thereafter, is a key component of secondary prevention. Aspirin, statin therapy, and an ACE inhibitor are recommended for all patients in the absence of contraindications. For patients with LV dysfunction, beta-blockers are strongly recommended, but uncertainty exists about their role in a successfully reperfused patient at low risk. If ACE inhibitors cannot be tolerated, angiotensin receptor blockers should be substituted and long-term aldosterone blockade added for patients with left ventricular dysfunction and an ejection fraction less than 40 %.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


