Transcatheter Aortic Valve Replacement
Saif Anwaruddin MD, FACC, FSCAI
Aortic valve stenosis (AS) is primarily a disease of the elderly, with the incidence increasing in patients over the age of 70. The presence of symptoms in the setting of severe aortic stenosis is known to portend a dismal prognosis (1). The standard of care for severe symptomatic aortic stenosis is surgical aortic valve replacement (AVR). However, many patients with severe symptomatic AS are not offered surgery. In fact, the Euro Heart Survey on valvular heart disease documented that 33% of patients above the age of 75 with severe symptomatic aortic stenosis were denied surgical valve replacement (2). The reason for this is believed to be the combination of advanced age and the presence of medical comorbidities.
Previously, the alternative to surgical AVR in patients deemed too “high risk” for surgery was balloon aortic valvuloplasty (BAV). Introduced in the mid-1980s, BAV as a therapy has been limited by high rates of both early and late restenosis (3, 4). BAV is a procedure in which the stenosed aortic valve is crossed with a straight wire in a retrograde fashion, and then exchanged for a stiff 0.035” guidewire upon which a 20- to 23-mm diameter balloon catheter is tracked up across the aortic valve. With the initiation of rapid pacing of the right ventricle, balloon inflation across the stenosed aortic valve is performed. In addition to high rates of restenosis, the procedural risks of BAV include embolic stroke, vascular complications, and severe aortic insufficiency. Currently the ACC/AHA guidelines limit the use of BAV to either cases of palliation or situations “to bridge” unstable patients to a more definitive therapy, such as AVR (5).
Until recently, the alternatives for patients with symptomatic critical aortic stenosis deemed inoperable or too high risk for open surgical AVR have been limited. With the advent of transcatheter AVR (TAVR), percutaneous approaches of replacing degenerative calcific aortic valves provide an option for those previously unable to undergo surgical AVR. This chapter discusses the procedure itself, the data supporting its use, and issues regarding the procedure. We will focus on the balloon expandable device, given its recent Food and Drug Administration (FDA) approval and availability of randomized data for it, but will also aim to provide a brief overview of the self-expanding valve experience.
TAVR: THE PROCEDURE
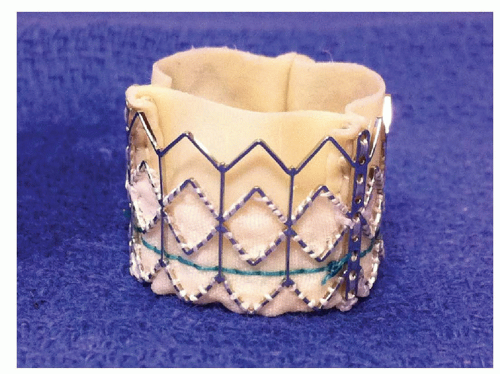
The recent publication of the Placement of Aortic Transcatheter Valves (PARTNER) trial (6, 7) and the FDA approval of the balloonexpandable Edwards Sapien transcatheter aortic valve system has provided a viable alternative for patients with critical aortic stenosis previously deemed inoperable (Fig. 41-1). The Medtronic Core Valve system is currently under investigation in the United States for this purpose as well (Fig. 41-2).
Preprocedural Evaluation
Paramount to the TAVR procedure is the preprocedural evaluation of the patient. This assessment should provide key information regarding the patient’s candidacy for TAVR. Currently, in the United States, patients with symptomatic critical aortic stenosis are eligible for TAVR only if they are deemed by a surgeon to be inoperable. Hence, a multidisciplinary approach is required for a complete evaluation of the patient. An evaluation should include an assessment of the severity of valve disease, the presence of any coronary artery disease and/or left ventricular dysfunction, the presence of concomitant medical comorbidities such as chronic obstructive pulmonary disease (COPD), or renal insufficiency, a thorough evaluation of the peripheral arteries for vascular access, and an assessment of the patient’s frailty.
This workup entails transthoracic echocardiography (TTE) and/or transesophageal echocardiography (TEE), cardiac catheterization for evaluation of coronary anatomy and hemodynamic parameters, pulmonary function testing, carotid duplex imaging, computed tomography (CT) angiography, and objective testing to measure frailty. CT has emerged as an imaging modality of choice for the preprocedural workup of the TAVR patient. In addition to obtaining accurate and reproducible measurements of the ileofemoral anatomy for access, CT provides valuable information regarding aortic valve annular dimensions, the presence of ascending aortic calcification (porcelain aorta), and the anatomy of the ascending aorta and related structures.
The presence of medical comorbidities needs to be addressed at the evaluation stage. The presence of any malignancy should be evaluated for short- and long-term prognosis. Decisions should also be made regarding the need for pre-TAVR coronary revascularization procedures.
Preprocedural evaluation of vascular access is an important step to determine suitability for a transfemoral (TF) approach for TAVR. Currently approved for commercial use in the United States, the Edwards Sapien valve is available in two sizes, 23 mm and 26 mm. Selection of valve size depends on the patient’s aortic
valve annular dimensions. The 23-mm valve requires a minimum ileofemoral diameter of 7 mm for a 22F sheath, and the 26-mm valve requires a minimum ileofemoral diameter of 8 mm for a 24F sheath. This is also dependent on vessel compliance as vessels can usually stretch on the order of 1 to 2 mm to allow for a larger sheath. If there is extensive circumferential calcification in the ileofemoral vessels, then the minimum diameter requirement increases for the respective valve sizes as this will affect vessel compliance (8).
valve annular dimensions. The 23-mm valve requires a minimum ileofemoral diameter of 7 mm for a 22F sheath, and the 26-mm valve requires a minimum ileofemoral diameter of 8 mm for a 24F sheath. This is also dependent on vessel compliance as vessels can usually stretch on the order of 1 to 2 mm to allow for a larger sheath. If there is extensive circumferential calcification in the ileofemoral vessels, then the minimum diameter requirement increases for the respective valve sizes as this will affect vessel compliance (8).
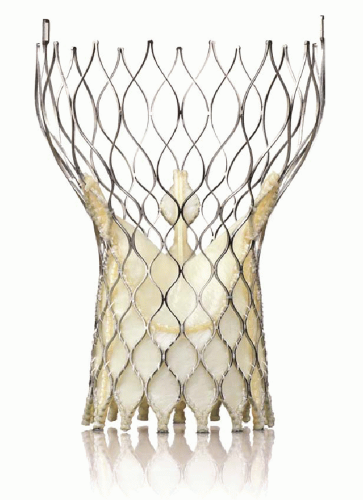 FIGURE 41-2 Medtronic self-expanding core valve transcatheter aortic valve. (Copyright Medtronic, Inc., Minneapolis, MN.) |
The Medtronic Core Valve system, currently under investigation in the United States, is available in a 26-mm and 29-mm valve size and delivered via an 18F sheath. A careful assessment needs to be made of the vessel size, angulation, calcification, and tortuosity in planning a strategy for access.
Procedural Imaging Modalities
Beyond the preprocedural imaging evaluation in the TAVR patient, intraprocedural multimodality imaging remains an essential component of the procedure. To have a working familiarity with these modalities and to be able to understand the anatomy of the aortic root complex in each of these modalities is necessary. Fluoroscopy/angiography, TEE, and CT are the most important imaging tools that are relied upon.
As mentioned previously, CT is used extensively in the preprocedural planning stages. It provides valuable information regarding annular dimensions, aortic root anatomy, vascular access, and vascular anatomy. This information is used to assess technical issues related to valve delivery and placement.
Fluoroscopy/Angiography is the mainstay of imaging used for all aspects of the transcatheter valve delivery, positioning, and deployment. It is not used in isolation, but echocardiography and CT are complementary. Fluoroscopy is used to carefully obtain vascular access and position the delivery sheath. In addition, fluoroscopy is used to identify aortic valvular calcification and to establish landmarks for valve positioning prior to deployment.
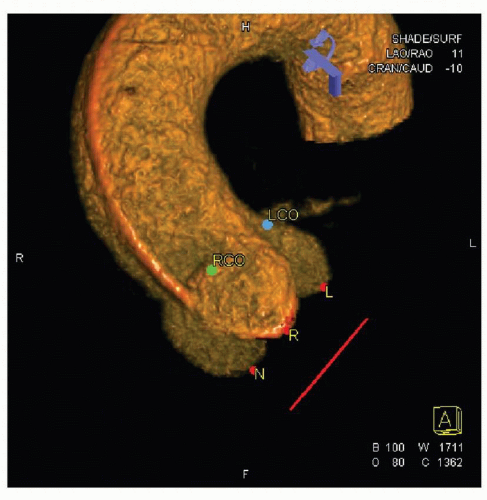
Selecting the optimal angiographic view is necessary for proper valve positioning. The view must identify the three aortic valve sinuses in a coplanar configuration, with minimal overlap with the spine so that the calcification of the aortic valve/annulus can be clearly visualized. The coplanar alignment of the sinuses allows for proper assessment of valve position prior to deployment. Three-dimensional rotational angiography performed prior to valve positioning can help with this process. Three-dimensional reconstruction of aortic root/valve complex can allow assessment of annular dimensions and distance between annulus and coronary ostia, and help determine optimal angiographic views for valve positioning (Fig. 41-3). This can be accomplished during the TAVR procedure itself or during the preprocedural catheterization. Three-dimensional rotational angiography requires less radiation than CT angiography and compares favorably to measurements obtained from TEE (9).
TEE during the procedure is important for defining valvular anatomy, annular sizing, and quantifying valvular regurgitation and ventricular function intraprocedurally. TEE is also helpful as an adjunct to fluoroscopy in valve positioning just prior to deployment. After deployment, TEE is necessary to accurately assess for either valvular or paravalvular (PVL) aortic regurgitation, ventricular function, mitral valve function, coronary ostial blood flow, and pericardial effusion or tamponade. An immediate assessment can be helpful in identifying a potential complication of the valve deployment.
Although all three imaging modalities ultimately serve to be complementary to one another during the procedure, in the planning stages a comparison of the imaging strategies (TTE, TEE, and CT) would suggest larger differences in annular measurements between CT and echo, ultimately impacting the clinical strategy (10). As such, even in the planning stages, the use of multimodality imaging to assess annular dimensions is likely more helpful than any one modality.
TRANSCATHETER VALVE DELIVERY, POSITIONING, AND DEPLOYMENT
For the purposes of this chapter, we will focus on the TF approach to TAVR (TF-TAVR). It is important to understand, however, that alternative approaches are available, depending on the valve itself. With the Edwards Sapien valve system, transapical delivery is possible through a left-sided mini thoracotomy, and the valve is delivered though the left ventricular apex under direct visualization and fluoroscopy. There is a separate delivery sheath for this purpose, but is not currently approved in the United States for commercial application. For the Medtronic Core Valve and for the Edwards Sapien valve, case reports of direct or transaortic delivery of the valve have been published (11, 12). Subclavian artery access is also feasible using the Core Valve system (13).
Necessary for the TAVR procedures is a hybrid operating room/catheterization laboratory that will allow for simultaneous fluoroscopy guided catheter manipulation and conversion to open surgery, if needed. It should also be large enough to accommodate a team consisting of cardiac anesthesiologists, echocardiographers, cardiac surgeons, and interventional cardiologists (14). An example of a hybrid room is shown in Figure 41-4A and B.
In the early experience of TF-TAVR, endovascular access was obtained via surgical cutdown to expose the common femoral artery, given the large sheath diameters. Currently, percutaneous access of the common femoral artery is the preferred mode of vascular access utilizing a previously described suture-based preclose method and crossover technique to obtain proximal vascular control (15). Once percutaneous access above the bifurcation of the common femoral artery into the profunda and the superficial femoral artery is obtained, the vessel can be serially dilated, and the valve sheath carefully inserted under fluoroscopic guidance. Anticoagulation should be initiated immediately after placement of the large valve sheath. Venous and arterial access is also obtained on the contralateral side for placement of a temporary transvenous pacemaker and a pigtail catheter, respectively.
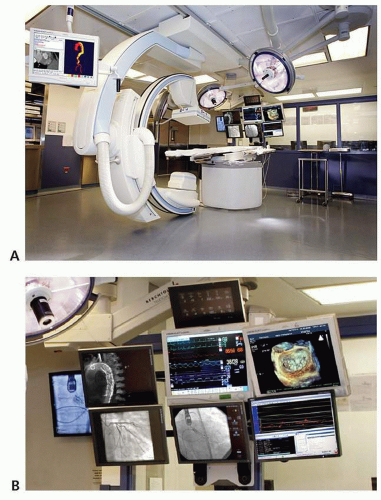 FIGURE 41-4 A: This picture demonstrates an example of a hybrid cardiac catheterization laboratory/operating room for TAVR procedures. B: Multimodality imaging display in the hybrid room. |
Once the desired view has been decided upon for valve positioning, a BAV is routinely performed prior to valve delivery so as to make room for the transcatheter valve inside the native stenosed aortic valve. It is important to be aware of the degree of aortic insufficiency present prior to BAV, as a sudden worsening of aortic insufficiency can sometimes lead to hemodynamic instability.
The delivery of the valve catheter is generally accomplished under fluoroscopic guidance and positioned across the native aortic valve. An aortogram can be performed to identify calcium landmarks and to assess overall valve position prior to deployment (Fig. 41-5




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree