risk factor modification, identified an annual mortality of 4% in patients with chronic stable angina (6).
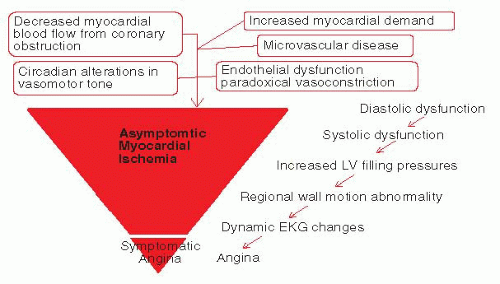 FIGURE 15-1 The iceberg effect of the ischemic cascade—the burden of asymptomatic myocardial ischemia and the tip of the iceberg—symptomatic angina. |
1. Identify and treat any associated medical conditions that may worsen or precipitate angina, such as thyrotoxicosis or anemia.
2. Modify established CAD risk factors and commence secondary preventative medications.
3. Modify lifestyle factors.
4. Commence, titrate, and ensure compliance with antianginal pharmacotherapy.
5. Perform revascularization (PCI or CABG) for persistent symptoms, or substantial residual ischemia on medical therapy.
In comparison, patients receiving contemporary medical therapy for stable CAD (92% were taking platelet inhibitors, 62% βblockers, and 58% lipid-lowering therapy) were evaluated in the EUROPA trial and had an annual risk of cardiovascular death or MI of 2.5% (40). Of the medical therapies available, aspirin, angiotensin converting enzyme (ACE) inhibitors, and lipid-lowering statin medications have been proven to reduce mortality and morbidity in patients with stable CAD and preserved left ventricular function. To avoid one death or MI, about 175 patients need to be treated with aspirin for 1 year (relative risk reduction: 23%); 120 patients with standard dose statin medications (relative risk reduction: 30%); and 200 patients with an ACE inhibitor (relative risk reduction: 20%) (1). The other medications, including longacting nitrates, βblockers, and calcium-channel antagonists, have been shown to improve symptomatology, exercise tolerance, and quality of life among patients with stable CAD; but their effect on survival has not been definitely established, with the exception of βblockers in patients with stable CAD and impaired left ventricular function (1, 10).
TABLE 15-1 Medical Therapies for Chronic Stable Angina | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
frequency and a 36% reduction in duration of ischemic episodes (1, 22, 41). Prior studies have examined the use of antianginal medications effecting combination for potential synergy. In the ACIP study, the combination ofβblocker and calcium-channel antagonist resulted in total ischemia suppression of 48% compared with the combination of calcium-channel antagonist and long-acting nitrate (total suppression 33%) (34). Current treatment guidelines recommend initial therapy with an antiplatelet agent and β blocker, with subsequent addition of a long-acting nitrate if symptoms persist. Calcium-channel antagonists are most commonly used in place of β blockers, and addition of a second agent is recommended if symptoms persist. Numerous additional antianginal agents are available for use, but a detailed discussion of these agents is beyond the scope of this chapter. They are listed in Table 15-1.
TABLE 15-2 ACC/AHA Recommended Pharmacotherapy for Chronic Stable Angina | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 15-3 Specific lifestyle goals in patients with chronic stable angina | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
with most therapy prior to this targeted toward ACS patients. The earlier trials of medical therapy for stable angina used different drug preparations and combinations, and different drug dosages and titration schedules (34). The sole lifestyle modification targeted was smoking cessation. It was not until the early 2000s that a greater emphasis was placed on β blocker therapy and ACE inhibitor use in most patients with coronary heart disease (10, 40). Successive treatment guidelines have broadened the indications for cholesterol reduction therapy, with endorsement of progressively earlier initiation of statin therapy and lower lowdensity lipoprotein (LDL) targets. Similarly, targets for blood pressure control have been serially reduced. Over time, a more significant emphasis has been placed on diet, exercise, weight control, diabetes management, and blood pressure control. In light of the evolution of medical therapy over time, it is difficult to compare the findings of multiple studies of medical therapy versus revascularization from different eras, or even to extrapolate the findings of prior studies to current practice, as therapeutic options, targets, and management strategies have changed so dramatically.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


