INDICATIONS/CONTRAINDICATIONS
Indications
Tracheoplasty via posterior splinting of the airway is performed in cases of severe tracheomalacia. Often the malacia, or dynamic airway collapse, involves not only the trachea but also the bronchial tree as well. In these cases of tracheobronchomalacia, tracheobronchoplasty is utilized to achieve stabilization of the entirety of the abnormal airways. While very focal malacia may be detected, most cases of acquired dynamic airway collapse involve both the trachea and bronchial airways. For purposes of this chapter the terms tracheomalacia and tracheoplasty will be used to encompass the full spectrum of disease and treatment of the central airways, including the trachea and bronchial tree.
It is crucial that the indications for surgical intervention are well understood. If not, the surgeon may be treating the anatomic and radiographic findings, but not the patient. The simple presence of tracheomalacia is not an indication for intervention. In addition, some degree of collapsibility is normal, with nearly three-quarters of healthy volunteers exhibiting cross-sectional airway reduction greater than 50% during maneuvers designed to increase intrathoracic pressures such as forced expiration or Valsalva.
The symptoms that may result from tracheomalacia include dyspnea, cough, orthopnea, and retained secretions. In addition, tracheomalacia may lead to recurrent infections, and, in extreme cases, respiratory failure. The presence of tracheomalacia and mild symptoms would not warrant intervention. The indication for tracheoplasty would be severe malacia coupled with significant symptomatology. Previous experience shows that nearly all patients undergoing tracheoplasty (94%) will have dyspnea as one of their symptoms. Nearly three-quarters of patients will suffer intractable cough preoperatively, and half will report a history of recurrent respiratory infections.
A note of caution in interpreting the indications for surgery is that all of these symptoms and associated conditions are nonspecific.
Contraindications
Contraindications to surgery are relative and include previous airway interventions such as resection or tracheoesophageal fistula repair, esophagectomy, or left pneumonectomy. Caution must be taken regarding the tracheal blood supply in cases of previous airway surgery. Left pneumonectomy would impair the ability to perform a right thoracotomy approach for the tracheoplasty. Other conditions leading to impaired pulmonary function and inability to tolerate prolonged periods of single lung ventilation should also be taken into account. While the operation can be performed with two lung ventilation and a retractor system to help keep the inflated right lung out of the field, it is not ideal, and significant hypoxia or carbon dioxide retention preoperatively makes this elective surgical intervention less attractive.
Relapsing polychondritis is a condition that may manifest as tracheomalacia in addition to other stigmata of cartilaginous abnormality. However, the dynamic collapse in this condition tends to be concentric, and thus, the posterior stabilization afforded by tracheoplasty tends to be ineffective. It would also be important to search for other conditions that may be more straightforwardly treated and may be related to the patient’s nonspecific symptoms, for example, atypical reflux with cough, IgG deficiency and recurrent respiratory infections, allergic rhinitis and postnasal drip with cough, or vocal cord dysfunction with cough and dyspnea.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
The preoperative planning for this operation can be thought of as three separate assessments.
1. Determining the degree of tracheomalacia
2. Eliminating confounding comorbidities
3. Predicting the likelihood of significant symptomatic relief from tracheoplasty
Establishing a diagnosis of tracheomalacia can be accomplished with dynamic airway computed tomography (CT) or functional bronchoscopy. Dynamic airway CT scanning has been shown to reliably identify airway collapse. The protocol for this involves scanning during a breath hold at total lung capacity and comparing the luminal patency to another series, which is obtained during a forced expiratory maneuver. The airway cross-sectional area is obtained by hand tracing the inner wall of the airway’s contour with an electronic tracing tool, and then the percentage of expiratory luminal collapse can be calculated (Fig. 40.1). Functional bronchoscopy involves fiberoptic endoscopy of the awake patient. Similar coached expiratory maneuvers are performed to estimate the degree of luminal collapse (Fig. 40.2). Concordance between these two diagnostic methods has been demonstrated. Nevertheless, both studies are utilized to assure that severe tracheomalacia is present. Air trapping, emphysema, or other parenchymal abnormalities may be detected additionally with CT scanning. Bronchoscopy may detect endobronchial abnormalities such as diverticula, tumor, or inflammation that might be missed radiographically.
The degree and the extent of malacia are determined by dynamic airway CT and functional bronchoscopy and are reported in standardized fashion as a percentage of luminal collapse during expiration at the proximal tracheal, distal tracheal, right mainstem bronchial, bronchus intermedius, and left mainstem bronchial levels. Additional information about the condition of the cervical trachea or the lobar and segmental bronchi is also reported. In general only patients with excessive expiratory collapse (>80% to 90%) would be considered for surgical stabilization.
While the etiology of most cases of acquired severe diffuse tracheomalacia remains unknown there are conditions that may confound the understanding of the impact that tracheomalacia may have on a patient’s symptomatology. Vocal cord dysfunction or paradoxical vocal fold motion is evaluated by endoscopic examination and if found may be treated with voice therapy or medications such as proton pump inhibitors, anxiolytics, gabapentin, or even botulinum toxin injection. In some cases patients who have symptoms of cough or breathlessness may have sufficient improvement to avoid the need for surgical airway stabilization. Gastroesophageal reflux disease (GERD) appears to be highly prevalent in the tracheomalacia patient population. Nearly 50% of patients who underwent tracheoplasty in the largest published series were also found to have GERD. While a causal link between GERD and microaspiration and the development or propagation of tracheomalacia has not been established, the possibility of GERD causing chronic cough is enough of a confounder that a formal pH study to evaluate for GERD is performed prior to intervention for the tracheomalacia. Some patients will undergo an antireflux procedure based on the degree of reflux detected, and in some cases tracheoplasty is rendered unnecessarily following this.
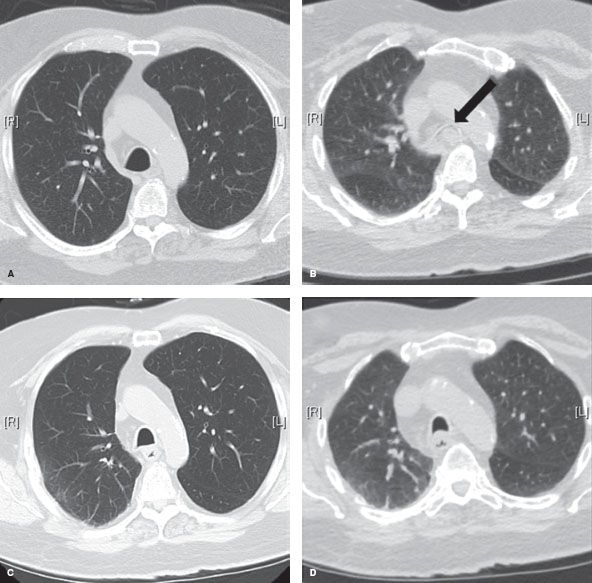
Figure 40.1 Dynamic airway CT images. A: Preoperative end-inspiration at distal trachea. B: Preoperative dynamic expiration at distal trachea. Arrow points to 100% collapsed airway lumen. C: Posttracheoplasty end-inspiration image at distal trachea. D: Posttracheoplasty dynamic expiration image at distal trachea.
Once a diagnosis of severe, diffuse tracheomalacia is established, an attempt is made to understand the potential salutary effects of airway stabilization. A stent trial is performed utilizing a Y-shaped silicone tracheobronchial stent, which is placed via rigid bronchoscopy and will achieve internal stabilization of the thoracic trachea and bilateral mainstem bronchi. After a 2-week trial, patients are seen in clinic to review the degree of improvement in symptomatology if any that might be attributed to stabilization of the central airways. Patients who reported marked symptomatic improvement would be considered for definitive surgical intervention.
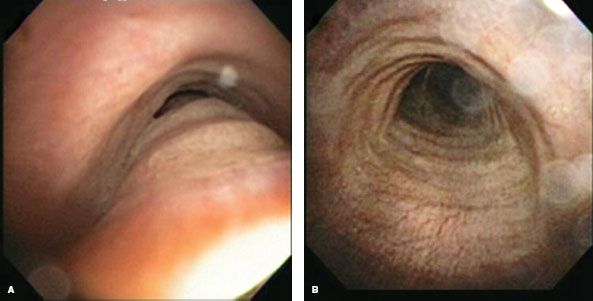
Figure 40.2 Functional bronchoscopy. A: Preoperative inspiratory image of the same patient as Fig. 40.1. B: Preoperative forced expiratory image. Note the near-complete obliteration of distal tracheal lumen.
Of the possible symptoms that internal stabilization with a stent might ameliorate, alleviation of dyspnea is the most consistent and obvious measure of treatment effect, partially because dyspnea is the most common presenting complaint, and partially because the stent has an immediate effect on expiratory airflow. The impact of stent stabilization on intractable cough may be more equivocal. The stent may alleviate the paroxysmal barking cough caused by severe expiratory airway collapse; however, the effect of a foreign body (stent) in the airway may in and of itself cause an aggravating cough and therefore limit the “readout.” The stent trial also may be limited because of a stent-related complication such as mucous plugging, granulation tissue formation, or infection. Judging the impact on symptoms may be difficult because of the negative symptoms generated by the stent itself or because the duration of stenting prior to its removal for adverse events is too short. The impact of a stent on the risk of recurrent respiratory infections is impossible to truly judge in a short duration as well.
A stent trial may not be possible to complete in patients with unusually large or small airways if correct stent sizing is not available, or in patients whose anatomy precludes safe rigid bronchoscopy. Given these limitations, it is important to attempt to tease out the beneficial outcomes of stent placement and consider them separately from the stent-related complications and adverse symptoms that might affect a majority of patients, peaking at the 3-week mark poststent placement. It is important to assess for the potential “placebo effect” of a stent. In addition, it is important that significant improvement is the benchmark. Slight improvement of symptoms should not constitute a positive stent trial. Despite the limitations, a planned short-term (<2 weeks) stent trial usually yields useful information about the likelihood of the patient with tracheomalacia to respond to airway stabilization. Between 60% and 75% of patients with tracheomalacia will respond to a stent trial in positive fashion and improvement in quality of life following definitive surgical airway stabilization with tracheoplasty is seen in 80% of these carefully selected patients.
Finally, the usual preoperative stratification of risk from cardiac or other comorbidities would be performed. Pulmonary function testing is performed and may reveal obstructive disease though this is a variable finding. Similarly flow-volume loop analysis may reveal normal patterns or a varying set of abnormal patterns. Moreover, the degree of expiratory tracheal collapse does not correlate with airflow limitation in patients with tracheomalacia. In general, though pulmonary function tests are obtained on all patients prior to intervention, a primary use for the results is to predict the likelihood of an arduous or complicated recovery from invasive intervention due to pulmonary comorbidity. Six-minute walk duration is measured preoperatively and poststabilization of the airway to assess the impact on functional status. In addition, determination of Karnofsky performance status, American Thoracic Society dyspnea score, and respiratory-affected quality of life based on the St. George’s Respiratory Questionnaire are done to ensure that significant impairment of a patient’s well-being exists prior to considering intervention for tracheomalacia.
 SURGERY
SURGERY
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


