INDICATIONS/CONTRAINDICATIONS
Indications
Aspergillus encompasses over 350 known species including Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, and Aspergillus terreus which are the most common pathogenic species. With increasing frequency, Aspergillus species are being recognized as an important cause of life-threatening infections in immunocompromised patients. The ever expanding population of immunocompromised patients includes patients with prolonged neutropenia, advanced human immunodeficiency virus (HIV) infection, and inherited immunodeficiency; patients having undergone hematopoietic stem cell transplantation and lung transplantation. Aspergillosis is now the third most common systemic fungal infection requiring hospitalization in the United States. Pulmonary aspergillosis was classified into allergic, invasive, and saprophytic infections in 1952 by Hinson et al. Clinical manifestations of pulmonary Aspergillus infection include:
1. A localized form, aspergilloma, which is an opportunistic infection most commonly found in individuals with pre-existing lung disease
2. Allergic bronchopulmonary aspergillosis, which is secondary to a complex immunologic response to exposure to noninvasive Aspergillus species
3. Disseminated aspergillosis, which occurs in immunosuppressed individuals
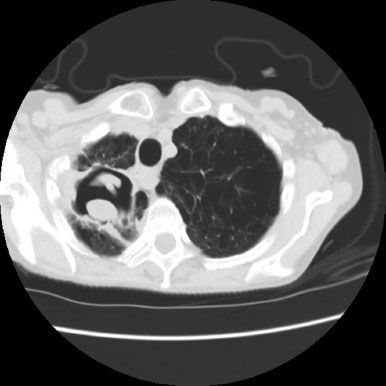
Pulmonary aspergilloma is the most common form of Aspergillus infection. Aspergilloma occurs when a pre-existing pulmonary cavity is colonized by the fungus producing a complex mass of septate hyphae, blood elements, and debris commonly referred to as a mycetoma or fungus ball. While the true incidence of aspergilloma is unknown, various cavitary lung diseases including bullous emphysema, fibrotic lung disease, histoplasmosis, sarcoidosis, and tuberculosis can be a predisposing factor in their formation.
It is generally believed that systemic antifungal agents are ineffective for aspergilloma. Surgical intervention for aspergillosis occurs in two distinct circumstances: Lung biopsy for the diagnosis of invasive aspergillosis in an immunocompromised patient and resection for complications of aspergilloma. There are no randomized controlled trials addressing the best treatment of aspergilloma. Literature focusing on aspergilloma treatment consists primarily of isolated case reports and retrospective case series. The major surgical indication for patients with aspergilloma is severe or recurring hemoptysis. Thirty percent of patients with even minor hemoptysis can progress to massive life-threatening hemoptysis with an associated 25% mortality. Daly et al. from our group in 1986 reported on the results of surgical treatment for pulmonary aspergilloma. Underlying chronic lung disease or immunologic risk factors were present in 92% of patients. The most common indication for operation was an indeterminate mass, hemoptysis, or chronic cough. In Daly’s series, all aspergillomas were classified as either simple or complex. Simple aspergillomas had thin-walled cysts with little surrounding lung disease. Complex aspergillomas had thick-walled cavities usually greater than 3 mm, with substantial surrounding lung disease and/or associated infiltrates. While in our series, the most common procedures performed were lobectomy (45%), pneumonectomy (17%), wedge excision (15%), and segmentectomy (11%); cavernostomy with muscle transposition was performed in six patients (11%). Postoperative complications were more common in patients with complex aspergilloma than among those with simple. It was noted that to reduce operative complications in some of our patients with complex aspergillomas, six were treated with cavernostomy and obliteration of the cavity with intrathoracic transposition of extrathoracic skeletal muscle. Others too have advocated cavernostomy with or without muscle flap transposition in patients with limited respiratory function or poor general condition as a safe method to effectively treat aspergilloma and prevent recurrence of hemoptysis.
Contraindications
The goal of aspergilloma surgery is limited resection resulting in removal of all diseased tissue sparing more normal lung parenchyma. For simple aspergilloma in patients with reasonable performance status and adequate pulmonary reserve, resection (e.g., wedge, segmental, lobectomy, rarely pneumonectomy) of the aspergilloma is the procedure of choice. Cavernostomy is an option for the management of peripheral complex aspergilloma in high-risk patients. The principal contraindications to cavernostomy are technical and relate to the location of the aspergilloma or the amount of afflicted lung. For example, very medially situated aspergillomas or those central within the lung far removed from the visceral pleural surface are not good candidates for cavernostomy.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
The natural history of aspergilloma may be highly variable; therefore, the initial management of a patient depends on the clinical presentation. While surgical resection is the preferred curative treatment for aspergilloma, cavernostomy with muscle transposition may be the best option for the most challenging higher-risk patients. Among these patients are those with severe hemoptysis and severe lung destruction with a high risk of morbidity. Cavernostomy may also be the most reasonable alternative in patients with more isolated peripheral aspergilloma but poor performance status and limited pulmonary reserve. Depending on the patient’s presentation, appropriate resuscitation is undertaken and respiratory support provided as needed. When a patient presents with mild non–life-threatening hemoptysis or cough, initial management can be conservative including humidified oxygen, cough suppression, and postural drainage. These patients require close follow-up. Flexible bronchoscopy should be performed to rule out more serious causes of airway bleeding or cough. In cases of severe hemoptysis, bronchoscopy should quickly be performed to identify the bleeding site. Intrabronchial instillation of iced saline with or without epinephrine may temporarily stop the bleeding. If the bleeding is massive, balloon occlusion of the bleeding airway may be helpful. Alternatively, double-lumen endotracheal tube intubation may be necessary for isolation of the bleeding lung and to protect the contralateral lung from aspiration of blood. Bronchial artery embolization may be helpful in initially stopping most bleeding. We often consider embolization a temporizing measure. Bleeding can recur in greater than 50% of cases due to the existence of an extensive array of collateral vessels. Embolization, however, may obviate the need for emergent surgical intervention in this high-risk population.
Mandatory prerequisites to successful cavernostomy command adequate lung debridement, closure of all bronchial openings, avoidance of contamination of the surrounding pleural space, and the ability to dependably transpose extraskeletal muscle intrathoracically. It is our strong belief that these essential items are most safely accomplished by the collaborative efforts of a thoracic and plastic surgeon. Intrathoracic muscle transposition requires the consideration of several factors: Location and size of the exposed cavity, general condition of the patient, condition of the transposed muscle (e.g., previous radiation or surgery), patient’s lifestyle, and type of work. The serratus anterior, latissimus dorsi, and pectoralis major muscles are all ideally suited for intrathoracic transposition. These muscles all have one major dominant vascular pedicle high in the thoracic inlet and of sufficient length to reach most intrathoracic locations. Furthermore, these muscles are of sufficient size to obliterate most pleural spaces. While not common, pectoralis minor and trapezius have also been utilized. Knowing which chest wall muscles are available at the time of cavernostomy and appropriately protecting these muscles during cavernostomy is important.
Nutritional status is important in this group of patients who are often debilitated and malnourished from chronic infection. If necessary, nutritional supplementation including liberal use of enteral feedings should be considered in all of these patients.
 SURGERY
SURGERY
There are four basic principles for cavernostomy: (1) adequate lung debridement, (2) avoidance of contamination of the remaining pleural cavity, (3) closure of all bronchial openings, and (4) obliteration of the residual pleural space. Our most common approach is two-staged. The first stage consists of limited chest wall resection, cavernostomy and removal of the fungus ball, suture closure of any bronchial openings followed by a series of wound pack changes. Only after successful completion of the first stage is the second stage undertaken, which specifically includes transposition of a muscle flap to obliterate the exposed cavity, an additional series of pack changes, and eventual definitive closure of the chest wall. Gebitekin et al. have reported on a small series of patients with complex aspergilloma successfully treated with single-stage cavernostomy and myoplasty.
All procedures begin with a team briefing. Surgeons, anesthesiologists, and operating room personnel are in attendance. Details of the airway management individualized for each specific patient are a major focus of the briefing. Patient identification, site marking, procedure verification, and final anesthetic evaluation are completed. Appropriate intravenous access is established. Epidural catheters are not utilized because of the risk of contamination. Instead, postoperative pain management most commonly is via a patient-controlled analgesia (PCA) pump. At least for the initial procedure isolation of the lungs is achieved by using a double-lumen endotracheal tube. Following intubation, a urinary catheter and lower extremity sequential compression devices are placed. Subcutaneous injection of 5,000 units of unfractionated heparin is administered to reduce the risk of deep venous thrombosis (DVT) and pulmonary embolism. All procedures are done in an open fashion, in a lateral decubitus position with the affected lung’s side up.
First Stage
Rib Resection, Cavernostomy, Closure of Bronchial Fistulae, Wound Packing
 The patient is positioned keeping in mind both the location of the aspergilloma and the extraskeletal muscle that will be transposed to eventually obliterate the cavernostomy (Figs. 9.1 and 9.2A,B).
The patient is positioned keeping in mind both the location of the aspergilloma and the extraskeletal muscle that will be transposed to eventually obliterate the cavernostomy (Figs. 9.1 and 9.2A,B).