Tracheal Resection and Reconstruction
Joel D. Cooper
The surgical management of both benign and malignant tracheal conditions is challenging, often daunting, but very rewarding because of the impact it can have on the daily life of the patient. Technical challenges are posed by both the resection and the reconstruction of the involved airway. The entire process is a logistic as much as a technical exercise, best conducted by an experienced team including radiologists, pulmonologists, thoracic surgeons, otorhinolaryngologists, thoracic anesthesiologists, skilled nurses, and respiratory therapists. The timing and the extent of resection are dictated as much by the presence or absence of comorbidities, as by the nature, location, and extent of the resection required. In carefully selected and wellprepared patients, tracheal resection and reconstruction offer a definitive treatment with excellent long-term outcomes.
Broadly speaking, there are three different types of tracheal resections, each requiring a specific operative approach and surgical technique depending on the location and extent of tracheal involvement. The most straightforward of these is a segmental resection of the trachea with end-to-end anastomosis. Resections at either end of the trachea, specifically a laryngotracheal resection at the proximal end, or a carinal resection at the distal end, may require more complicated anastomotic reconstruction and complementary release maneuvers to reduce tension.
SURGICAL ANATOMY OF THE TRACHEA
The trachea is a more or less “D”-shaped tube which, in the adult, is of fairly uniform caliber connecting the larynx above with the main bronchi below. It is supported by c-shaped cartilaginous rings and a nonsup-ported posterior membranous wall. The elastic properties of the cartilaginous portion provide for some degree of lengthening, such as when the head is extended, and the mobile, nonreinforced posterior membranous wall allows a certain degree of change in caliber in response to changes in pleural pressure. The trachea begins in the neck as an anterior cervical structure and terminates distally as a posterior mediastinal structure. Its blood supply is primarily a segmental one with branches from the inferior thyroid artery, the internal thoracic artery, and the bronchial arteries along with variable contributions from the subclavian and brachiocephalic arteries. The blood supply enters the trachea along either lateral aspect from which the vessels travel horizontally between cartilaginous rings. Because the blood supply to the trachea comes primarily from multiple lateral end vessels, it is essential not to circumferentially devascularize the trachea for more than 1 to 2 cm. However, complete blunt anterior and posterior dissection is well tolerated for purposes of mobilization without consequent ischemia to the trachea.
Along its entire posterior aspect, the membranous wall of the trachea is adjacent to the esophagus. Along the anterior surface of the trachea, the thyroid lies at the level of the cricoid and first tracheal ring. The left brachiocephalic artery crosses anteriorly at a variable position, which may be as high as 1 to 2 cm above the sternal notch, or a similar distance inferior to the sternal notch. The right azygos vein lies adjacent to the right lateral aspect of the distal trachea at or just above the tracheobronchial angle. The aortic arch lies in the left anterolateral position to the distal trachea, and the pericardium lies immediately anterior to the carina. The left recurrent laryngeal nerve lies in the tracheoesophageal groove throughout the length of the trachea, while the right recurrent laryngeal nerve occupies a similar position on the right side from the level of the right subclavian artery proximally. Both nerves ascend to the larynx posterior to the cricoid lamina. All of these anatomic relationships have significant implications for the approach and technique used for mobilization and resection of the trachea. In addition, in older patients with increasing kyphosis, a loss of tracheal elasticity and possible calcification may all have significant implications for the approach and extent of safe tracheal resection.
ETIOLOGY OF POTENTIALLY RESECTABLE TRACHEAL LESIONS
Indications for tracheal resection and reconstruction include both benign and malignant conditions with benign conditions far outweighing the malignant ones. The most common indication is postintubation injury to the airway followed by inflammatory conditions especially idiopathic subglottic stenosis, which occurs primarily in young women. Currently, tracheal resection for Wegener’s granulomatosis, relapsing polychondritis, amyloidosis, and other inflammatory conditions is exceedingly rare.
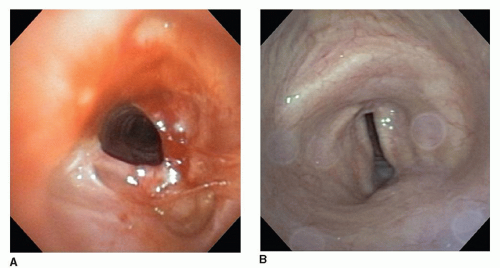
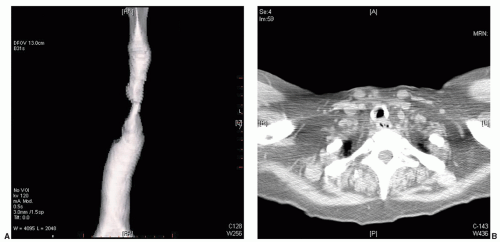
Postintubation tracheal stenosis remains the most frequent indication for tracheal resection, though the spectrum of postintubation injuries has shifted in recent years. Formerly, postintubation stenosis at the cuff site of endotracheal tubes or tracheostomy tubes was the most common source of injury followed by stomal stenosis due to loss of the supporting cartilaginous arch at the tracheostomy stoma site. In recent years, however, postintubation stenosis at or just below the cricoid cartilage has become more common and represents a more complex problem. These injuries are usually caused by high placement of a tracheostomy tube with subsequent damage and infection at the level of the cricoid cartilage often extending proximally to within 1 cm of the vocal cords. This injury may be associated with total obliteration of the airway in the subglottic region leaving the patient dependent on a tracheostomy tube and unable to speak (Fig. 9.1). The reason for this increasing number of high airway strictures is uncertain, but the use of percutaneous tracheostomies with malpositioning of the tracheostomy site, urgent posttraumatic placement of a tracheostomy tube in patients who are now more likely to survive complex trauma
and the rising incidence of morbid obesity making even elective tracheostomy more difficult to site at a desirable position at the second or third tracheal ring all may contribute.
and the rising incidence of morbid obesity making even elective tracheostomy more difficult to site at a desirable position at the second or third tracheal ring all may contribute.
Among malignant conditions requiring tracheal resections, adenoid cystic carcinoma remains the most common. Resection for primary squamous cell carcinoma of the trachea is uncommon both because of the rarity of the condition and the commonly advanced stage of the tumor at the time of diagnosis in addition to improved results with radiotherapy as an alternate option. Tracheal resection for secondary involvement of the airway with malignant tumors arising in other sites is limited almost exclusively to resection associated with direct invasion by thyroid carcinoma.
Whatever the indication, appropriate selection of patients and judicious selection of timing of resection are essential in producing a satisfactory result. Evaluation of both the location of the lesion as well as the extent of the resection required is critical in making a decision as to whether resection is the optimal treatment and if so the type of resection required. In addition, some thought also must be given to the use of temporizing maneuvers to delay resection until the patient is in an ideal condition. The use of silicone stents such as T-tubes frequently aides in preoperative management, and the use of permanent stents as an alternative to resection may, in some cases, prove to be a reasonable strategy.
CLINICAL PRESENTATION AND DIAGNOSTIC STUDIES
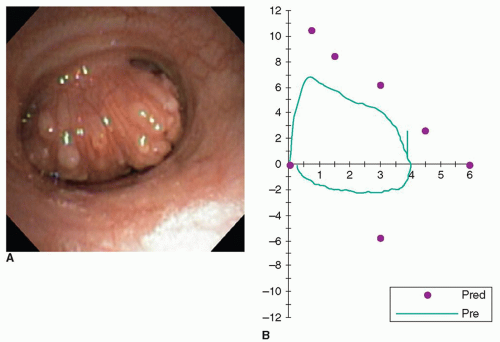
The presenting symptoms of patients with tracheal obstruction vary greatly depending on the etiology of the airway compromise. Postintubation stenosis usually presents relatively early following the hospital course, which necessitated ventilator assistance in the first place. If the tracheal damage is proximal usually caused either by a traumatic intubation or injury to the cricotracheal junction from an endotracheal tube or a highly placed tracheostomy stoma, airway obstruction may first be recognized by the inability to decannulate the patient because of an inadequate upper airway. More often postintubation cuff stenosis generally presents as increasing shortness of breath with onset 4 to 8 weeks after decannulation when the full thickness damage to the trachea from the resulting inflammation and fibrosis leads to circumferential scarring and contraction (Fig. 9.2A). An exception is stomal stenosis whereby loss of the anterior supporting portion of the trachea, either due to too large a tracheostomy stoma or extension of the stoma by leverage on the tracheostomy tube by unsupported connection between the ventilator tubing and the tracheostomy, results in collapse of the lateral walls. This so-called inverted V or A-frame stenosis may first present years or even decades after the tracheostomy tube was removed (Fig. 9.2B).
Thyroid carcinoma invading the trachea may be suspected either because of stridor suggesting airway compromise or, for lesser invasion, discovered at
the time of thyroidectomy. If there is any question ideally, a bronchoscopy should be performed prior to undertaking the thyroidectomy. Squamous cell carcinomas of the trachea may present with hemoptysis or with a gradual increase in breathlessness or stridor especially with exertion. Adenoid cystic carcinomas that are usually quite slow growing may present with a history of several years of gradually increasing dyspnea on exertion ultimately resulting in shortness of breath with routine activities. A history of increasing breathlessness with stridor going back five years or more is not uncommon.
the time of thyroidectomy. If there is any question ideally, a bronchoscopy should be performed prior to undertaking the thyroidectomy. Squamous cell carcinomas of the trachea may present with hemoptysis or with a gradual increase in breathlessness or stridor especially with exertion. Adenoid cystic carcinomas that are usually quite slow growing may present with a history of several years of gradually increasing dyspnea on exertion ultimately resulting in shortness of breath with routine activities. A history of increasing breathlessness with stridor going back five years or more is not uncommon.
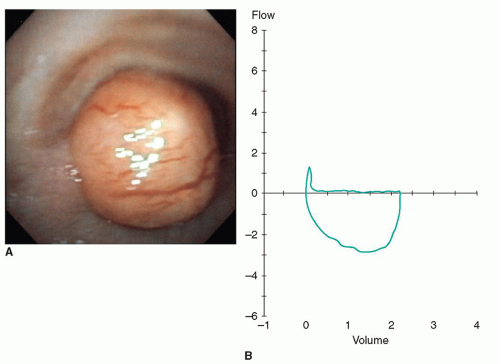
Furthermore, the degree of airflow obstruction is influenced by the location as well as the physical size of the tumor. This is illustrated in Figure 9.3A and 9.3B.
Benign tumors of the trachea may also present with a very long history of gradually increasing exertional dyspnea. Often these patients have had an extensive work-up for asthma-like symptoms. These tumors, which ultimately may fill the tracheal lumen, often are attached at a very small base or stalk, leaving the rest of the tracheal wall uninvolved. Because of the ability of the intrathoracic trachea to expand on inspiration during normal respiration, such patients may not be noticeably short of breath but may be found to have an FEV1 of <10% of predicted because forced expiration compresses the trachea against the tumor causing the near total obstruction.
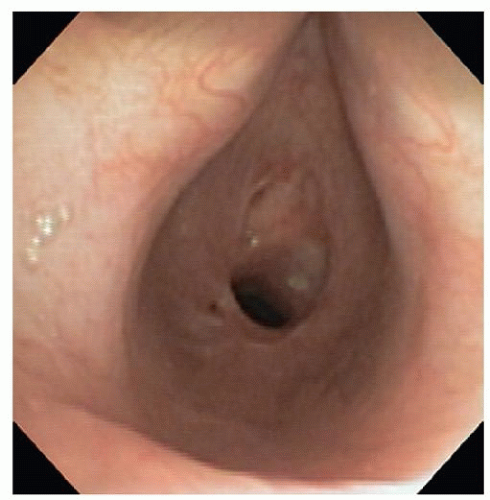
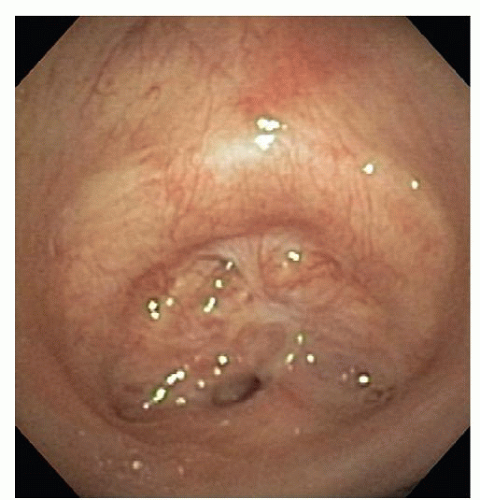
The diagnosis of postintubation stricture is usually apparent from the history and confirmed by bronchoscopic evaluation. The diagnosis of both benign and malignant tumors is also usually obvious on the basis of radiographic and bronchoscopic assessment. Conversely, the diagnosis of idiopathic subglottic stenosis is usually made based upon the typical presentation in females, with gradual progression of stenosis and the exclusion of other benign conditions such as previous intubation, Wegener’s granulomatosis and relapsing polychondritis (Fig. 9.4). The diagnosis is subsequently confirmed with bronchoscopy.
ASSESSMENT OF THE EXTENT OF AIRWAY INVOLVEMENT AND RESECTABILITY
Routine preoperative assessment includes a carefully obtained history, physical examination, bronchoscopic evaluation, and pulmonary function studies in addition to imaging. For patients who do not have a tracheostomy tube in place, computed tomography (CT) imaging usually precedes bronchoscopic evaluation. For patients with a tracheostomy in place, the reverse is true and at the time of CT imaging the best study is obtained if the tracheostomy tube can be temporarily removed. This involves determining in advance whether or not the patient can maintain a satisfactory airway for 30 to 60 seconds without the tracheostomy tube in place. If so, the CT scan is done with a physician in attendance. Once the patient is positioned for the scan, the tracheostomy tube is removed and immediately reinserted after the scan is completed. It is best to practice this maneuver with the patient before proceeding with the actual scan. When possible the CT scan should be done with the acquisition of images in both inspiration and expiration. Evaluation of the CT images is best done with a three-dimensional reconstruction along with the assessment of both the axial and sagittal slices.
Depending on the exact software used for the CT analysis a three-dimensional reconstruction usually defines quite nicely the location and the extent of the narrowing of the airway column. However, it does not demonstrate the actual wall thickness or consistency and it is for this reason that the evaluation of the axial sections in particular is essential. It is not uncommon for the airway wall above and below the narrowed segment to be extensively involved with calcification and scarring. In such a situation, the extent of resection will be determined not only by the length of narrowed airway but also by
the amount of tracheal wall involvement proximal and distal to it, which may have considerable influence on the ability to reconstruct the airway if one resects back to normal airway at either end. Based upon the radiologic and endoscopic assessment, a decision can be made as to the resectability as well as choice of operative approach, and the likelihood of release maneuvers that may be required in order to assure a satisfactory, relatively tension-free primary anastomosis (Fig. 9.5). The circumstances most likely to produce scarring, thickening, and calcification include trauma to the neck region, one or more previous tracheotomies, inhalation injury, or the presence of a postintubation tracheoesophageal fistula. Scarring of the airway wall beyond the segment of actual stenosis may provide significant technical difficulty at the time of resection. This is due to the loss of mobility of the airway and loss of tracheal elasticity, which ordinarily help to reduce tension on the anastomosis. Calcification makes it difficult to place sutures through the wall, and mediastinal scarring leads to fixation of the airway and difficulty in mobilization to help bring the severed margins of the resected airway together. Previous sternotomy, obesity, and kyphosis also influence resectability, the surgical approach, and the likelihood of a favorable outcome.
the amount of tracheal wall involvement proximal and distal to it, which may have considerable influence on the ability to reconstruct the airway if one resects back to normal airway at either end. Based upon the radiologic and endoscopic assessment, a decision can be made as to the resectability as well as choice of operative approach, and the likelihood of release maneuvers that may be required in order to assure a satisfactory, relatively tension-free primary anastomosis (Fig. 9.5). The circumstances most likely to produce scarring, thickening, and calcification include trauma to the neck region, one or more previous tracheotomies, inhalation injury, or the presence of a postintubation tracheoesophageal fistula. Scarring of the airway wall beyond the segment of actual stenosis may provide significant technical difficulty at the time of resection. This is due to the loss of mobility of the airway and loss of tracheal elasticity, which ordinarily help to reduce tension on the anastomosis. Calcification makes it difficult to place sutures through the wall, and mediastinal scarring leads to fixation of the airway and difficulty in mobilization to help bring the severed margins of the resected airway together. Previous sternotomy, obesity, and kyphosis also influence resectability, the surgical approach, and the likelihood of a favorable outcome.
BRONCHOSCOPIC ASSESSMENT
Bronchoscopic evaluation by the surgeon is an essential step in determining the management and resectability of a tracheal or laryngotracheal lesion. This assessment is usually done beginning with flexible bronchoscopy following nasopharyngeal topical anesthesia with the patient mildly sedated so as to be able to assess vocal cord function. Following this, the flexible bronchoscope is advanced through the larynx. If the patient does not have critical airway stenosis, the level of sedation can be increased after assessment of the vocal cords and flexible bronchoscopic assessment continued with or without the use of a laryngeal mask to help support ventilation. Should preoperative or initial bronchoscopic visualization suggest the presence of a markedly narrowed airway in the absence of tracheostomy tube in place, a decision must be made whether or not to traverse the narrowed segment with the flexible bronchoscope out of concern for causing edema or bleeding, which could lead to a critical situation. In this circumstance, the presence of a skilled thoracic anesthesiologist, a skilled nursing team, and a wide variety of equipment appropriate for the situation is essential. This includes rigid bronchoscopes in sizes ranging from 5 mm upward, flexible bronchoscopes ranging from pediatric to therapeutic in size, appropriate-sized dilating balloons, and equipment for jet ventilation through the open, rigid bronchoscopes. It is important to avoid muscle relaxants until a safe airway has been established. This maybe greatly facilitated by the use of a laryngeal mask and the use of Dexmedetomidine (Precedex) sedation, which is relatively unique in its ability to provide sedation without causing respiratory depression. Its use is ideal in the patient requiring awake, rigid bronchoscopic dilatation of the critically narrowed airway.
Until recently, the author favored initiating dilatation of a critical airway stenosis with a No. 5 rigid pediatric bronchoscope converting to general anesthesia once the initial passage of the bronchoscope beyond the stricture had been accomplished. Increasingly, however, the use of a dilating balloon through the therapeutic bronchoscope, which itself is passed through a laryngeal mask, has proven very satisfactory and is somewhat less demanding of the skills and experience of the thoracic surgeon and anesthesiologist. The one concern with initiating dilatation with a balloon rather than with a rigid bronchoscope has been that any bleeding or edema might convert the critical airway to an obstructed airway necessitating an emergent tracheostomy. With the use of a dilating balloon having a diameter in the 6 to 8 mm range, this has
not been a problem and provides increased safety of the airway for the subsequent dilatation maneuvers. Generally, this would set the scene for converting to a general anesthesia including, if necessary, the use of paralytic agents, for further dilatation using rigid bronchoscopic techniques. The use of progressively larger rigid bronchoscopes undoubtedly provides a better dilatation than the use of progressively larger dilating balloons even if their inflated diameter is equal to or greater than that of the rigid bronchoscopes selected for dilatation (Fig. 9.6).
not been a problem and provides increased safety of the airway for the subsequent dilatation maneuvers. Generally, this would set the scene for converting to a general anesthesia including, if necessary, the use of paralytic agents, for further dilatation using rigid bronchoscopic techniques. The use of progressively larger rigid bronchoscopes undoubtedly provides a better dilatation than the use of progressively larger dilating balloons even if their inflated diameter is equal to or greater than that of the rigid bronchoscopes selected for dilatation (Fig. 9.6).
After the narrowed segment of trachea has been dilated, the airway should be reassessed with the flexible bronchoscope to determine the stability of the airway. Ideally, the dilatation procedure should lead to a more secure upper airway, eliminating the need for urgent tracheostomy or surgical intervention while further assessment of both the airway and the patient’s suitability for resection is considered.
TIMING OF SURGICAL INTERVENTION
The appropriate timing of a tracheal resection is one of the most important factors in producing a satisfactory outcome. This is especially true for benign lesions as many temporizing maneuvers can be used to delay surgical resection until the patient’s condition is optimal. These temporizing procedures may include repeated dilatation, use of a silicone T-tube stent, or even prolonged use of a tracheostomy tube to provide a safe airway.
As previously noted, most tracheal resections are currently done for postintubation strictures or malacia of the airway. In many cases, the airway compromise is recognized initially within a couple of months of the initial intubation, when the patient has not completely recovered from the condition (trauma, cardiac surgery, acute respiratory failure, etc.), which necessitated intubation in the first place. In such patients, it is advisable to temporize until the patient is fully active and has achieved their maximum possible overall recovery. This often means delaying definitive resection for a year or more. Any patient with potentially reversible medical conditions should have resection deferred until such time as they are deemed best equipped from a cardiopulmonary standpoint, have attained the best possible physical conditioning, and any ongoing steroid requirements for persistent underlying lung disease have been reduced or eliminated. The need for postoperative ventilatory assistance following a tracheal resection should be assiduously avoided as it is usually associated with an unsatisfactory result. Reoperation after a failed tracheal resection is clearly associated with increased risk, complexity, and an inferior result compared with an initial, successful reconstruction.
Most cases of critical stenosis can be managed for 7 to 10 days with a single dilatation using rigid bronchoscopy, after which a plan for long-term management should be formulated. If internal tracheal stents are to be used, only silicone stents or T-tubes should be utilized since expandable metal stents may cause further mucosal damage and complicate the subsequent surgical resection. If a silicone T-tube is employed, it should be used only if the patient can breathe comfortably with the horizontal (external) limb capped except for instillation of saline and mucolytic agents. If the patient is unable to breathe comfortably with the horizontal limb capped, then the upper, vertical limb will become clogged with mucous and it is preferable that a tracheostomy tube rather than a T-tube be utilized. If the patient presents with upper airway obstruction and no tracheostomy stoma is present, the stoma for a temporizing tracheotomy tube, or T-tube should be made through the damaged portion of the airway if at all possible. This will avoid damaging an additional segment of the trachea, which further complicates a subsequent resection and reconstruction.
If airway compromise, whether from a benign or malignant condition, is the patient’s primary problem and the patient is otherwise suitable for resection, it still maybe advisable to improve the airway, whether by dilatation of the stricture or by endoscopic partial resection of an obstructing tumor to allow the patient to improve their exercise tolerance and improve secretional clearance to further minimize the likelihood of a postoperative pulmonary infection. Usually 3 to 4 weeks of such preparation suffices. If the patient presents with a tracheostomy tube in place, then the use of a silicone T-tube placed through the tracheostomy stoma to stent the narrowed segment of airway is often the optimum method of temporizing and can be in place for an indefinite period. In some situations, leaving a T-tube in place for 6 to 9 months before its removal may lead to a stable widely patent airway, thus eliminating the need for a resection. In the majority of such cases, however, temporary stenting alone is not sufficient and a resection is usually required (Fig. 9.7).
PRINCIPLES OF TRACHEAL RESECTION
As previously noted, tracheal resections can, in general, be divided into three anatomic categories: those requiring a segmental trachea resection with end-to-end to tracheal anastomosis; those involving a resection of the upper trachea and cricoid region with a resulting laryngotracheal anastomosis; those with resection of the distal trachea including the carina with anastomosis between the distal trachea and one or both main bronchi. This latter resection is usually indicated for malignant tumors and is dealt with in Chapter 10.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree