■ CLI with tissue loss often occurs in the setting of multilevel arterial occlusive disease. In the case of isolated diabetic tibial occlusive disease, femoral, and frequently popliteal, pulses remain palpable. In either circumstance, limb-threatening ischemia may ensue. In the latter circumstance, multilevel approaches to complete revascularization, either staged or simultaneous, should be pursued.
■ Neurovascular exam, with particular focus on the wound location and the extent of tissue loss, should be evaluated and documented. Probably, the most deterministic variable is the extent of tissue loss—Wagner wound classification, the presence and severity of osteomyelitis, exposure or involvement of the calcaneus bone, residual intact skin on either the dorsal or plantar foot. These conditions all impact decision making and clinical outcome.
■ Patient functional capacity also plays an important role in the therapeutic strategy. Options and outcome goals vary substantially between ambulatory and nonambulatory patients.
IMAGING AND OTHER DIAGNOSTIC STUDIES
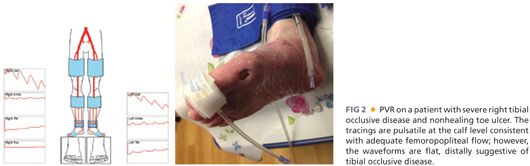
■ Pulse volume recordings (PVRs) (FIG 2)
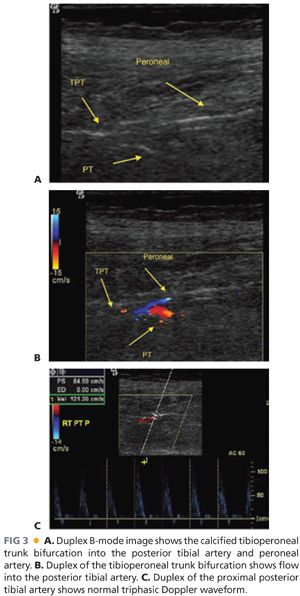
■ Duplex (FIG 3)
■ Computed tomography (CT) and magnetic resonance (MR) angiograms can be obtained; however, their diagnostic use in planning tibial interventions is frequently limited by the imprecision of bolus timing with distal extremity cross-sectional imaging techniques, heavy medial calcification frequently present in target arteries, and the diminutive size of reconstituted target arteries, which may be present in the peri- and inframalleolar regions.
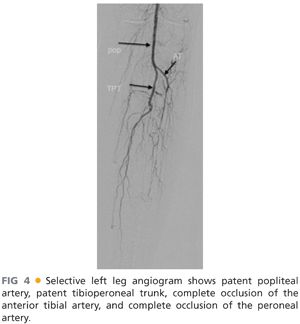
■ Catheter-directed, intraarterial angiography remains the gold standard imaging study for tibial occlusive disease for both diagnostic and therapeutic purposes (FIG 4).
SURGICAL MANAGEMENT
■ Technical skills, careful planning, and knowledge of the relevant arterial anatomy determine tibial revascularization strategies for limb salvage. Current controversies include the potential value of restoring patency in more than one tibial vessel to optimize blood flow and maximize the chances of wound healing. Proponents of this approach reference the “angiosome” concept of the foot or the idea that specific skin regions derive primary perfusion from end-arterioles arising primarily from either the dorsal pedal or posterior tibial arteries as they cross the ankle. This practice is pursued in marked contradistinction to the open surgical imperative to restore in-line flow to the foot in the single largest, most continuous crural artery. The many advantages of endovascular reconstruction techniques in tibial reconstruction include restoring partial flow in multiple target arteries as compared to a single artery following surgical bypass, as well as opportunities to repeat procedures with relatively simple outpatient interventions as needed, to maintain patency and skin integrity. Treatment decisions regarding revascularization strategy in individual circumstances should be guided by patient-specific anatomic considerations, arterial runoff into the foot, patient habitus and ambulatory status as well as patency and feasibility considerations related to either open or endovascular options.
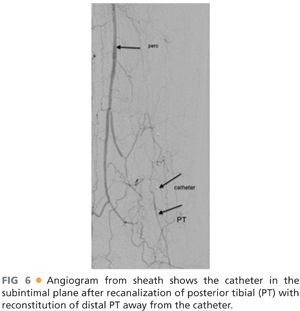
■ Currently available endovascular technology facilitates successful treatment of complex occlusive lesions at and below the malleolar level. Technical limitations remain, however, highlighted by risks of arterial perforation (FIG 5), difficulty in true lumen reentry in complete occlusions (FIG 6), procedure-related distal arterial embolization, and limited pedal vessel outflow in certain circumstance.

■ The retrograde or SAFARI (SubintimAl Flossing with Antegrade-Retrograde Intervention) tibial intervention technique may improve technical results in challenging lesions, particularly those resistant to ipsilateral antegrade access, including flush occlusions at the origin of the target artery or with large collateral arteries adjacent to the occluded origin. In nearly every circumstance, even chronic and recalcitrant occlusions may be crossed more easily from the retrograde rather than antegrade approach; this is true regardless of the chronicity of the lesion in question, degree of calcification, or length of occlusion.
Preoperative Planning
■ Preoperative vein mapping prior to the diagnostic angiogram is helpful in handicapping potential surgical alternatives and determining the extent to which interventional alternatives to be pursued.
■ Patients should be medically optimized prior to their procedure: Preventive strategies are advised to reduce the risk of kidney injury in patients at risk for contrast nephropathy; smoking cessation is encouraged as well as antiplatelet and statin therapy.
■ Tibial interventions can entail significant radiation exposure. Protective shields, lead glasses, and judicious use of fluoroscopy are recommended to protect all participants in the procedure. Ultrasound-guided access can minimize radiation exposure, particularly for pedal access; needle extenders allow the operator to puncture remotely and minimize hand exposure.
■ Micropuncture and pedal access kits are essential access tools.
■ Sheaths: 5 and 6 Fr, braided, 90 cm or 110 cm from contralateral femoral access; 45- to 55-cm sheath from the ipsilateral transfemoral access
■ Wires: 300-cm, 0.014-in or 0.018-in wires; 260-cm, 0.035-in floppy Glidewire™
■ Catheters: 150- to 170-cm catheters and balloons
■ Medications: heparin (or other anticoagulant), clopidogrel, nitroglycerin, papaverine, alteplase, and calcium channel blockers. Consider preprocedural perioperative antibiotics prior to procedures potentially requiring prosthetic implants.
Positioning
■ The patient is placed supine on the angiographic table with both groins prepped and draped. Consider preparing the foot and the leg in anticipation for retrograde approach if needed. Stockinette can be placed over the involved foot and the leg is covered with the angiographic drape (FIG 7).

TECHNIQUES
ANTEGRADE TIBIAL REVASCULARIZATION
First Step: Femoral Access and Anticoagulation
■ Contralateral femoral access with standard up and over technique is our routine approach for diagnostic arteriograms and most tibial interventions.
■ Antegrade femoral approach has distinct advantages for tibial or pedal interventions, especially in the setting of a hostile and narrow aortic bifurcation or occluded or severely diseased contralateral iliofemoral system. Antegrade access generally provides easier pushability and reduced radiation to the patient and procedural team.
■ Ultrasound-guided access may minimize risk for access site complications.
■ Diagnostic arteriogram to image the inflow is performed.
■ A 5- or 6-Fr sheath is advanced over a stiff wire to the popliteal artery, positioned as close as possible to the tibial trifurcation. (Sheath: 90 to 110 cm from the contralateral femoral access and 45 to 55 cm from the ipsilateral femoral access) (FIG 8).
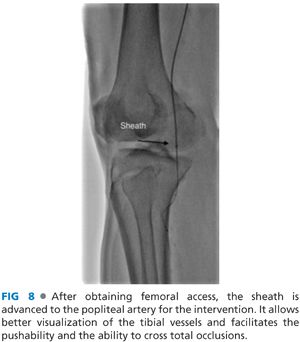
■ Sheath tip positioning close to the target vessel maximizes pushability across total occlusions. Also, improved visualization of tibial vessels is achieved with reduced contrast volumes.
■ Anticoagulation is established using unfractionated heparin or other alternatives to achieve an activated clotting time (ACT) of more than 250 seconds.
Second Step: Selective Angiogram
■ Imaging of the tibial outflow is obtained from sheath injections or through diagnostic catheters (5- to 10-mL power or hand injection). To reduce contrast load, contrast may be diluted 50% for all but the most distal arterial beds.
■ Anteroposterior or ipsilateral anterior oblique projections are obtained to visualize the popliteal “trifurcation” and separate the tibia and fibula. True lateral oblique projections are obtained to visualize pedal outflow.
■ Arteriographic images must be carefully examined to optimize outcome; multiple projections may be required to sufficiently opacify tibial and pedal vascular anatomy, especially distal to extended or serial occlusions. Delayed views (prolonged digital subtraction angiography [DSA] time) may improve opacification of patent tibial or pedal vessels distal to occluded segments (FIG 9). Withdrawing the sheath to the femoral bifurcation may uncover reconstitution of distal tibial artery segments through extended deep femoral artery collateral pathways.

Third Step: Crossing the Occlusion
■ Angled catheters and guidewires are typically used to select the respective tibial arteries.
■ The catheter/guidewire combination is advanced into the target tibial artery proximal to the occlusion or stenosis.
■ Anatomy is confirmed with magnified arteriographic views.
■ “Road mapping” may improve guidance across occlusions. The wire leads through the occlusion, followed by the crossing catheter (e.g., Quick-Cross™ or Cook CXI™, 0.014 in or 0.018 in) (FIG 10).
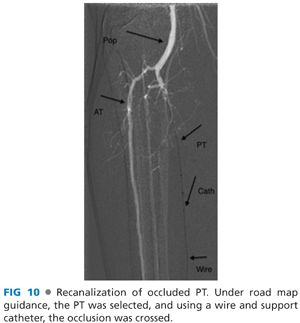
■ Transluminal passage is preferred to subintimal access, because reentry into the true lumen may be unpredictable and challenging.
■ Soft-tipped hydrophilic guidewires are used to negotiate and traverse tibial stenosis with the support of crossing catheters under magnified road map guidance.
■ Heavier weighted tip, chronic total occlusions (CTO) guidewires (either 0.014-in or 0.018-in platforms) are designed to provide improved performance and penetration across total occlusions.
■ For longer occlusions, leading with a 2-mm percutaneous transluminal angioplasty (PTA) balloon as an alternative to low-profile crossing catheters (e.g., Quick-Cross™ or CXI™) can improve access by extending or reestablishing the recanalization plane during transit.
Fourth Step: Reentry into the True Lumen
■ Reenter into the true lumen under road map guidance (FIG 11).
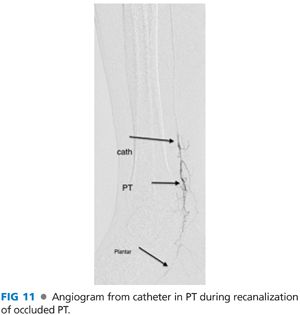
■ Advance the catheter over the wire into the true lumen beyond the target lesion and remove the wire.
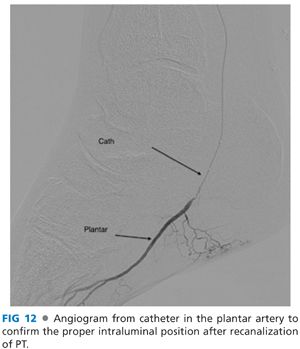
■ Aspirate to check for back-bleeding and subsequently perform a selective angiogram through the catheter to confirm the proper intraluminal position (FIG 12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


