■ Device designs vary with aneurysm extent. For pararenal aneurysms, 70% of patients are adequately treated with two small fenestrations for the renal arteries and a scallop for the superior mesenteric artery (SMA).5 Suprarenal and type IV TAAAs typically require four fenestrations (no scallops). Extensive TAAAs (types I to III) need directional branches, particularly if the aortic diameter is relatively large or aneurysmal at the level of the visceral arteries. The combination of directional branches for celiac and SMA management with fenestrations for the renal arteries is increasingly popular.
SURGICAL MANAGEMENT
Ancillary Tools
■ These procedures require advanced endovascular skills and a comprehensive inventory of applicable catheters, balloons, and stents (Table 1). Dedicated training in fenestrated and branched techniques is highly recommended for physicians already experienced in endovascular disease management and ancillary procedures including renal and visceral artery disease management.
Perioperative Measures
■ Patients with difficult aneurysm anatomy, chronic kidney disease, or advanced age are preadmitted for bowel preparation and intravenous hydration with bicarbonate infusion. Oral acetylcysteine is administered to minimize risk of periprocedural renal dysfunction following administration of iodinated contrast.
■ Hybrid, fixed imaging platforms are essential for optimal results of these complex procedures. Most are performed using general endotracheal anesthesia; local or regional anesthesia may be sufficient in select cases.
■ Intraoperative blood salvage systems (“cell saver”) are recommended for difficult cases and all TAAAs. The creation of large, impermeable pockets within dependent portions of the surgical drapes will facilitate pooling and collection via the cell saver.
■ The use of iodinated contrast is minimized by avoidance of power injector digital substraction angiography (DSA) runs during device implantation and side stent placement. Whenever possible, hand injections of dilute contrast (70% saline) are used to locate the side branches. Completion aortography is obtained only after all stents are positioned and postdilated, again using diluted contrast (50%).
■ To minimize contrast, precatheterization of targeted visceral arteries or use of onlay computed tomography (CT) images, when available, is recommended. In experienced hands, precatheterization adds little to the overall procedure time.
Positioning
■ Patients are positioned supine with the imaging unit oriented from the head of the table. Both arms are tucked for repair of pararenal aneurysms requiring up to three fenestrations.
■ Brachial artery access is used in patients treated by directional branches or those who need four fenestrations. The left arm is abducted and prepped in the surgical field up to the axilla. A working sterile side table is oriented in the same axis of the abducted arm for optimal support of necessary wires and catheters.
■ Electrocardiogram (EKG) leads, urinary catheter, and other monitoring cables and lines should be taped or secured so that they are not in the path of the x-ray beam of the fluoroscopic unit and do not impede movement of the C-arm gantry.
Arterial Access
■ Access is established in the femoral arteries. Patients with small, calcified, or stenotic iliac arteries may require creation of an iliac conduit for safe device delivery.
■ Total percutaneous femoral access is the preferred approach in patients with noncalcified arteries or mild posterior plaque. The standard “preclose” technique enables complete hemostasis in more than 95% of patients irrespective of sheath diameter.7 When femoral arteries are small, calcified, or bifurcate close to the inguinal ligament, standard surgical exposure and access is obtained. Proximal and distal control is obtained using vessel loops.
■ The left brachial artery is surgically exposed via small longitudinal incision in the upper arm, just proximal to the origin of the deep brachial artery.
■ Intravenous heparin (80 to 100 units/kg) is administered immediately after femoral and brachial access is established. An activated clotting time longer than 250 seconds is maintained throughout the procedure with frequent rechecks every 30 minutes. Prior to deployment of the stent graft, diuresis is induced with intravenous mannitol and/or furosemide.
TECHNIQUES
ENDOVASCULAR REPAIR USING FENESTRATED STENT GRAFTS
■ Fenestrated–branched repair is currently performed using the Cook Zenith® stent graft lineage. Newer designs by Endologix (Ventana), Terumo (Anaconda), and Cook Medical (p-Branch) are under clinical investigation.
■ The Cook Zenith® fenestrated stent graft consists of a proximal fenestrated tubular component, a distal bifurcated universal component, and a contralateral iliac limb extension (FIG 1A). The fenestrated tubular component is custom-made to fit the patient’s anatomy. Four to 6 weeks are required for manufacturing and delivery in the United States.
■ Bilateral percutaneous femoral access is established under ultrasound guidance; each femoral puncture is preclosed using two Perclose devices. Bilateral 8-Fr sheaths are introduced to the external iliac arteries over Benson guidewires (Cook Medical, Bloomington, IN). The guidewires are exchanged to 0.035-in soft glidewires and Kumpe catheters, which are advanced to the ascending aorta and exchanged for stiff 0.035-in Lunderquist guidewires (Cook Medical, Bloomington, IN).
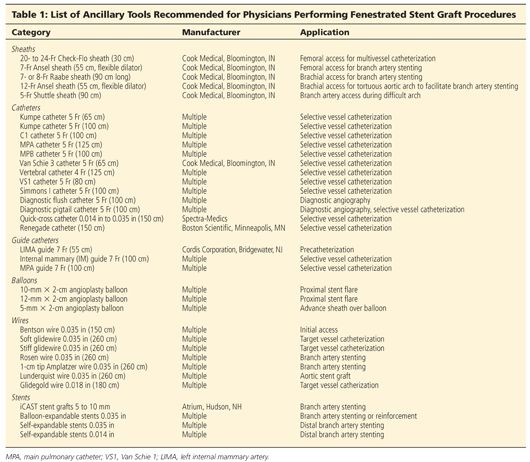
■ Choice of access site is dependent on tortuosity and vessel diameter. Provided there are no issues with both iliac arteries, the branches are performed via the right femoral approach, whereas the fenestrated and bifurcated devices are introduced via the left femoral approach. A 20-Fr (two fenestrations) or 22-Fr (three fenestrations) Check-Flo sheath (Cook Medical, Bloomington, IN) is introduced via the right femoral approach (FIG 2A). The valve of the Check-Flo sheath has four leaflets, which are accessed by two short 7-Fr sheaths at 2 o’clock and 7 o’clock positions.
■ Precatheterization of the renal arteries is performed using 0.035-in soft glidewires and 5-Fr Kumpe or C1 catheters (Cook Medical, Bloomington, IN), which are supported by 7-Fr left internal mammary artery (LIMA) guide catheters (FIG 2B). Alternatively, onlay fusion CTA is recommended to minimize contrast use.
■ Once the target vessels are catheterized, the fenestrated stent graft is oriented extracorporeally, introduced via the left femoral approach, and deployed with optimal apposition between the fenestrations and the target catheters.
■ Proper device orientation, using the anterior and posterior markers, is essential. It is useful to deploy the first two or three stents and then rotate the imaging unit laterally, confirming alignment between the catheter and its respective fenestration. The device should be deployed slightly higher than what is anticipated, with the catheter matching the lowest of the four radiopaque markers in the fenestration. The diameter-reducing wire on the fenestrated component allows for some rotational and cranial–caudal movement to optimize alignment following initial deployment.
■ After deployment of the fenestrated component, each catheter is removed from its target artery and used to sequentially regain target vessel access through the respective fenestration. (FIG 2C). In most cases, when alignment is carefully confirmed prior to attempted cannulation, the target vessel is accessed without difficulty.
■ After the target vessel is catheterized, soft glidewire is removed and hand injection is used to confirm location. The glidewire is exchanged for a 0.035-in Rosen guidewire (Cook Medical, Bloomington, IN). The Rosen guidewire has a floppy J tip, reducing the risk of branch renal artery perforations. When additional support is required, the Amplatz guidewire (Cook Medical, Bloomington, IN) with 1-cm soft tip can be used.
■ After the Rosen or stiff guidewire of choice is positioned, a 7-Fr Ansel sheath with flexible dilator is advanced. If there is difficulty to advance the sheath, an undersized balloon may be used as a dilator to facilitate advancement.
■ Once the sheath is in position, an alignment stent is positioned under protection of the sheath with the tip of the stent just beyond the tip of the sheath (FIG 2D).
■ For repairs requiring two or three vessel fenestrations, the target vessels are accessed sequentially using femoral approach. For those requiring four fenestrations, the celiac axis is accessed via brachial approach using a preloaded catheter, which is placed through the celiac fenestration and exits the stent graft via an access scallop at the top of the device.
■ The diameter-reducing tie on the fenestrated segment is removed after all the target arteries are accessed and secured by 7-Fr hydrophilic sheaths.
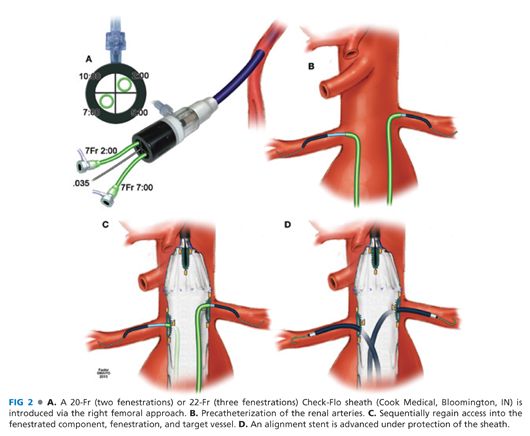
■ The top cap of the device is advanced forward to deploy the uncovered fixation stent (FIG 3A). The top cap is retrieved prior to deployment of the alignment stents.
■ After the top cap and dilator are removed, the proximal landing zone is gently dilated using a compliable balloon such as the Coda balloon (Cook Medical, Bloomington IN, FIG 3B). It is critical that the balloon dilatation is performed prior to placement of alignment stents, or alternatively, each stent has to be protected by separate balloons.
■ The alignment stents are sequentially deployed following removal of the diameter-reducing tie, retrieval of the top cap, and balloon dilatation of the neck. The sequence of stent deployment is renal arteries followed by SMA and celiac axis. Prior to each stent deployment, the position of the stent is confirmed by hand injection. The stent is deployed 3 to 5 mm into the aorta (FIG 3C) and flared using a 10-mm × 2-cm balloon (FIG 3D). A completion angiography of each branch is performed using hand injection after direct injection of 100 to 200 μg of nitroglycerin to minimize spasm.
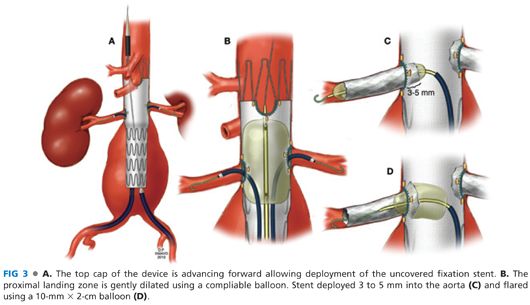
■ Following placement of the alignment stents, a distal bifurcated stent graft is oriented, advanced, and deployed with preservation of the ipsilateral internal iliac artery. The dilator of the bifurcated device may encroach the contralateral renal stent or the SMA stent. In these cases, it is useful to leave a 10-mm balloon ready to be inflated in the renal stent to prevent damage (FIG 4A, inset). The minimum overlap between the bifurcated and the fenestrated component is two full-length stents (17 mm each), but ideally, more than three full stents is recommended to minimize risk of component separation (FIG 4B).8 After deployment of the bifurcated device, the dilator is removed with care to avoid damage or dislodgement of the renal stents.
■ The contralateral gate is catheterized using a soft glidewire and 5-Fr catheter (FIG 4B). Access is confirmed by 360-degree catheter rotation. The glidewire is exchanged for a 0.035-in Lunderquist guidewire. Limited iliac angiography using contralateral oblique views with hand injection. The contralateral limb extension is deployed with preservation of the internal iliac artery (FIG 4C).
■ A completion angiography of the aorta and iliac arteries is obtained using power injection to demonstrate patency of the visceral arteries, main body, iliac limbs, and iliac arteries.
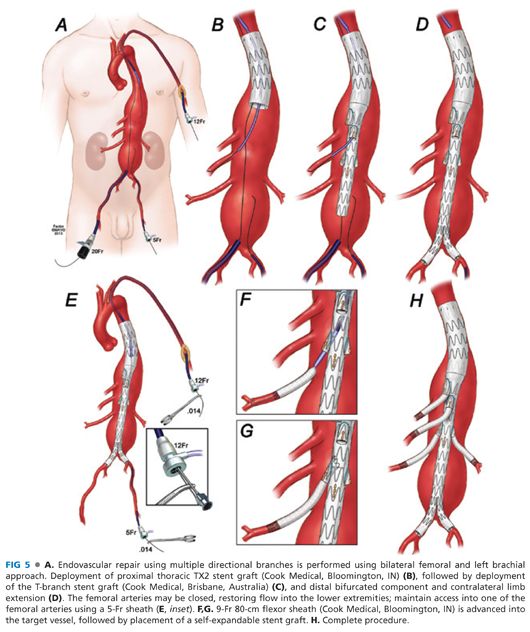
ENDOVASCULAR REPAIR USING MULTIPLE DIRECTIONAL BRANCHES (MULTIBRANCH T-BRANCH STENT GRAFT)
■ Directional branches created with presewn cuffs are currently available from Cook Zenith® stent graft lineage on an investigational-use basis (FIG 1B). A four-vessel multibranch stent graft design (T branch) is also being investigated for treatment of TAAAs.9
■ The extent of repair varies depending on the proximal extension of aneurysm within the thoracic aorta. The procedure is performed using bilateral femoral and left brachial approach. In general, the repair starts with deployment of a proximal thoracic TX2 stent graft (Cook Medical, Bloomington, IN) followed by deployment of the T-branch stent graft (Cook Medical, Brisbane, Australia) and distal bifurcated component and contralateral limb extension. The self-expandable stents are placed into the four branches following deployment of all aortic components. The critical steps are reviewed as follows:
■ Bilateral femoral and left brachial arterial access is obtained (FIG 5A). A proximal thoracic stent graft is deployed if needed depending on aneurysm extent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


