Thymic Tumors
Thomas W. Shields
Thymic tumors present almost exclusively in the anterior mediastinal compartment. Mullen and Richardson,205 in their collective review, reported that in the adult, thymic lesions accounted for 47% of masses in this compartment. In the total of 2,412 patients in a combined series, thymic lesions were second only to neurogenic lesions in total number of all mediastinal tumors and cysts in the adult. However, it is the opinion of most that, at present, the number of thymic lesions seen annually in adults exceeds the number of neurogenic lesions seen. These tumors are rare in children <16 years of age. However, Ramon y Cajal and Suster253 as well as Pescarmona246 and Kaplinsky127 and their colleagues, have reported a number of these tumors in children and have presented their histologic and immunochemical features.
There has been much controversy as to the classification of thymic lesions, particularly of the various tumors seen. In the latter twentieth century, one of the most accepted classifications was that of Rosai and Levine272 (Table 188-1). However, it would be appropriate from both clinical and pathologic viewpoints to exclude those tumors that do not arise from the thymic epithelium or the neuroendocrine cells located in the thymus. Thus, tumors of germ cell or lymphoid origin and metastatic lesions to the thymus are excluded in the present discussion. Likewise, thymic hyperplasia (Chapter 163) and thymic cysts (Chapter 204) are excluded in this consideration of thymic tumors. It is to be noted, however, that a thymoma may grossly present as a cyst and that a thymic tumor may develop within an established cyst. Moreover, a truly workable classification should be based either on the histologic features or the gross features of the lesion but not on a combination of both. Consequently, in this discussion, thymic tumors are separated into four basic categories: epithelial cell tumors (thymomas and thymic carcinomas), tumors of neuroendocrine origin, thymolipomas, and a miscellaneous group of rare tumors (neuroblastoma and ganglioneuroblastoma, malignant melanoma, thymic hemangiomas, and myoid tumors) (Table 188-2).
Epithelial Cell Tumors
Thymomas
Location
Marchevsky and Kaneko177 state that about 95% of all epithelial cell tumors of the thymus (thymomas) occur in the anterior compartment of the mediastinum. They have been found outside the confines of the mediastinum (a) in the neck, as reported by Ridenhour,264 Fukuda,80 and Hsu116 and their colleagues; (b) in the left hilar region, as reported by Cosio-Pascal and Gonzalez-Mendez46; and (c) within the pulmonary parenchyma, as reported by McBurney,183 Yeoh,371 Kung,147 Moran,195 and Veynovich342 and their associates. Within the mediastinum, thymomas also have been described to be located in the middle (visceral) compartment by Kojima138 and Kanzaki126 and their coworkers as well as in a supradiaphragmatic position by Perera and Wilson,242 by the present author and colleagues,289 and recently by Carr and O’Keefe.34 Cooper and Narodick44 reported one located in the visceral compartment extending into the paravertebral sulcus. al-Salem2 recorded the presence of thymic tissue in the posterior mediastinum. Von Wadon343 reported a thymoma that presented as an intratracheal polyp. Thymomas also have been reported to be rare primary tumors of the pleura (Chapter 70). Recently, Miller and associates188 described two thymoma-like lesions within a right and a left atrial myxomas in two elderly patients. It should be noted that thymic tissue had been reported earlier by Burke and Virmani30 to be present in cardiac myxomas.
Pathologic Features
All thymomas are derived from the thymic epithelial cells. The epithelial components may be identified by several immunohistochemical techniques using reaction with various antibodies (see Table 163-2 in Chapter 163).
According to McKenna and colleagues,185 only a small percentage of thymomas (about 4%) consist of a pure population of thymic epithelial cells. All the others contain varying numbers of other cells and lymphocytes. As a consequence, thymomas historically have been divided primarily into four histologic subgroups, including the spindle cell epithelial variant. Bernatz22 and Lewis159 and their associates suggest the lesions be classified as (a) predominantly lymphocytic thymoma when the tumor is made up of more than 66% lymphocytes; (b) predominantly epithelial thymoma when the epithelial cells represent 66% or more of the cell population; (c) mixed lymphoepithelial thymoma when neither of the aforementioned criteria is met; and (d) spindle cell tumor, which is a subtype of the epithelial variety and is separated from the latter by the histologic characteristics of the epithelial cells.
Table 188-1 Classification of Tumors of the Thymus | ||
|---|---|---|
|
This relatively simplistic classification was found to be inadequate to classify satisfactorily many of the epithelial tumors as to their origin and immunohistochemical properties. As a result, the histologic classification of the thymic epithelial tumors has undergone numerous changes during the last several decades of the twentieth century and the initial years of the twenty-first. One aspect, however, on which all investigators agree is that only the thymic epithelial cells (cortical and medullary) take part in the malignant behavior of thymomas that occur in the various cell types. The thymic lymphocytes may vary greatly in number in the various tumors observed, but these lymphocytes take no part in the neoplastic transformation that results in the formation of the various thymic epithelial tumors. The lymphocytes in most thymomas are small and mature-appearing, without evidence of cytologic atypia. Variations in cell pattern are observed; however, no evidence of malignant change is evident. Mokhtar and associates,190 by studying the reactions of the lymphocytes in thymomas to monoclonal and polyclonal antiserum markers, concluded that these cells mirrored the lymphocytic phenotypes of the normal thymus.
Table 188-2 Classification of Thymic Tumors | |
|---|---|
|
It had been an article of faith that all thymomas were composed of cytologically benign cells regardless of their benign or malignant behavior (i.e., invasion beyond the capsule of the tumor or the presence of local or distant metastasis). However, as early as 1987, Lewis and associates159 identified the presence of varying degrees of cellular atypia in 2% of the patients with otherwise typical benign thymomas. Similar and even more advanced atypical changes were subsequently noted in the malignant variants as well by Shimosato,293 Hishima and colleagues,109 Suster and Moran,311 and Kuo and Chan.149 In addition to these increasing sites of atypia, areas of actual carcinoma were also identified in a small number of patients. These latter thymomas were most often of the cortical histogenetic type associated with areas of either well-differentiated or poorly differentiated squamous cell carcinoma. Suster and associates313 also described a thymoma with pseudosarcomatous stroma that simulated a carcinosarcoma. Most of these combined thymomas with areas of thymic carcinoma (atypical thymomas) behaved clinically more like typical thymomas than like the very aggressive thymic carcinomas that were easily identified. Nonetheless, these atypical lesions appeared to provide evidence of a continuum of recognizable neoplastic change from the benign-appearing thymic epithelial tumors to the obviously typical malignant tumors. Thus, a perfectly normal-appearing (benign) thymic epithelial cell within a thymic tumor has, in fact, the potential for malignancy.
The nature of the epithelial cell components in the epithelial thymic tumors varies greatly. They may be either medullary or cortical cells in origin and may morphologically represent cells that are present in a mature or involuted gland. The organotypic features may likewise vary in their likeness to either a mature or involuted gland. As a rule, the epithelial cell component of the thymomas consists of large round, oval, or spindle-shaped cells. These tend to be grouped in clusters. The nuclei are vesicular with small nucleoli, and the cytoplasm is either eosinophilic or amphophilic with indistinct cell borders. The spindle cells may resemble fibroblasts and form whorls and fascicles; the epithelial nature of these cells has been established by ultrastructural studies and immunohistochemical techniques (see Chapter 163). Other microscopic features, as noted by Marchevsky and Kaneko177 and Kornstein,145 may be observed in these lesions: rosettes, pseudoglands, glands, papillary structures, Hassall’s corpuscles, myoid cells, keratinizing squamous epithelium, perivascular spaces, cysts, fibrous bands, squamous or medullary differentiation, and germinal centers. Each of these features occurs in varying percentages of these tumors, but other than the problem of occasional difficulties in differential diagnoses, these findings are of no major significance. Hofmann and Otto113 point out that the thymic epithelial cells can be arranged along blood vessels and give the appearance of a hemangiopericytoma; the spindle variety of the epithelial cell can grow in whorls or in a storiform pattern and may resemble a malignant fibrous histiocytoma; rosette and glandular formation may mimic a metastatic adenocarcinoma. The true nature of thymic epithelial derivation of the cells and increased certainty of the tumor being a thymoma can be detected by immunohistochemical tissue-staining techniques with the use of antikeratin antibodies, as described by Battifora17 and Löning167 and their colleagues. Hofmann and associates111,112 have described the various techniques of these immunohistologic studies in detail. Hirokawa and associates107 have reported the incidence of positive results of the various thymic epithelial markers in 45 thymomas. Cytokeratin was positive in 100%, thymosin beta in 89%, thymosin alpha in 80%, Th-3 mouse thymic nurse cells in 78%, Leu-7 in 67%, and UH-1 (cortical epithelium of human thymus) in 60%. They concluded that about 85% of the thymomas were of cortical epithelial cell origin (see Chapter 163). This is in essential agreement with the studies of Marino and Müller-Hermelink,178 who reported 55 of 58 thymomas (95%) to be of cortical epithelial cell origin. Kornstein and colleagues144 reported positive reactivity to cyto- keratin in 94%, epithelial membrane antigen in 77%, and Leu-7 in 80% of thymic epithelial cell tumors studied. Some reactivity occurred to neuron-specific enolase, but little to no reactivity was seen to carcinoembryonic antigen or chromogranin.
As the result of these many immunohistochemical studies, Müller-Hermelink and colleagues206,207,208 suggested a histologic classification based on the presence of either medullary or cortical phenotypic differentiation in a given thymoma. They classified the thymomas as (a) medullary, (b) mixed, (c) predominately cortical (organoid), (d) cortical, and (e) well-differentiated thymic carcinomas. The medullary thymomas are related phenotypically to the medullary epithelial cells, and cortical thymomas are related to the cortical cells. The mixed tumors showed components of both types. Using this scheme, Ricci and colleagues263 observed that most patients with the medullary type had stage I disease, whereas most with the cortical type had stage III or IV disease as defined by the staging system of Masaoka and coworkers.179 The medullary thymomas were composed of small- to medium-sized cells with irregular, often spindle-shaped nuclei devoid of nucleoli and low to moderate numbers of lymphocytes of the mature T-cell type. The capsule was thick, but intratumoral fibrous septa were usually absent. Atypia was absent, and mitotic activity was low. In the mixed thymoma, two epithelial components were present, one of which was that found in a medullary thymoma and the other of which was lymphocyte-rich, mimicking cortical areas of the normal thymus. The organoid thymoma was predominantly cortical and showed a recapitulation of both cortical and medullary areas of the normal thymus gland, including the presence of Hassall’s corpuscles. A complete fibrous capsule was frequently lacking. These organoid tumors had a high lymphocyte count and only a few epithelial cells were evident; the epithelial cells were elongated and mimicked the cells of the normal corticomedullary junction. The cortical thymomas were lobulated tumors with interlobular fibrous septa and often an incomplete capsule. The epithelial cells were medium to large in size with round to oval nuclei that contained dispersed chromatin, a prominent central nucleolus, and ill-defined cytoplasm. Squamous cell differentiation was often present at the periphery of the tumor. Lymphocytes were abundant and blastic in appearance. The well-differentiated thymic carcinomas that were included in the classification are no longer considered to belong in the thymoma group of tumors and are discussed subsequently. This classification was suggested to have independent prognostic implications—a concept supported by Wilkins,355 who noted only a few recurrences in patients with medullary or mixed tumors, whereas the recurrence rate was high in the cortical lesions. Rendina,261 Ricci,268 Pescarcoma,245 and Quintanilla-Martinez251 and their associates have also supported this classification. On the other hand, the studies of Shimosato293 and Regnard and colleagues257 did not agree with this classification or with its potential prognostic value; they noted invasion, even death, in patients with medullary-type tumors. The impression of Kornstein and associates146 and of Kornstein147 was that this classification was no more useful than the traditional classification.
In 1996308 and subsequently in 1999,312 Suster and Moran presented a simplified conceptual approach to the classification of thymic epithelial tumors, as follows:
Thymoma: a well-differentiated thymic epithelial neoplasm characterized by a population of round or spindle-shaped epithelial cells without cytologic atypia intimately admixed with lymphocytes in various proportions. The thymoma resembles many or all of the architectural organotypic features of the normal mature (childhood) thymus or features that recapitulate the normal involuted thymus of the adult.
Atypical thymoma: a moderately differentiated thymic neoplasm that is characterized by a predominantly epithelial cell population showing a moderate degree of atypia but retaining some of the organotypic features of thymic differentiation.
Thymic carcinoma: a poorly differentiated thymic epithelial neoplasm characterized by overt cytologic features of malignancy with total loss of organotypical features of differentiation of the normal thymus.
At about the same time, however, the World Health Organization (WHO) came out with a histologic classification
(resembling the Müller-Hermelink classification) as the classification of choice at that time. Rosai and Sabin276 published the WHO’s classification in the second edition of WHO, Histological Typing of Tumors of the Thymus (Table 188-3). Dadmanesh and associates50 described this classification succinctly: “In this schema, thymomas are evaluated on the combined basis of the morphologic appearance of the neoplastic epithelial cells … and the relative number of these cells vis á vis the nonneoplastic lymphocytes.” Type A stands for “atrophic,” representing the thymic cells of adult life; type B stands for “bioactive,” representing the biologically active thymus of the fetus and infant; and type C stands for “carcinoma.”
(resembling the Müller-Hermelink classification) as the classification of choice at that time. Rosai and Sabin276 published the WHO’s classification in the second edition of WHO, Histological Typing of Tumors of the Thymus (Table 188-3). Dadmanesh and associates50 described this classification succinctly: “In this schema, thymomas are evaluated on the combined basis of the morphologic appearance of the neoplastic epithelial cells … and the relative number of these cells vis á vis the nonneoplastic lymphocytes.” Type A stands for “atrophic,” representing the thymic cells of adult life; type B stands for “bioactive,” representing the biologically active thymus of the fetus and infant; and type C stands for “carcinoma.”
Table 188-3 The New World Health Organization Histologic Classification of Thymic Epithelial Tumors | ||
|---|---|---|
|
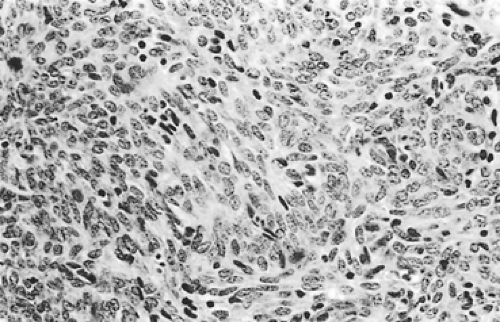
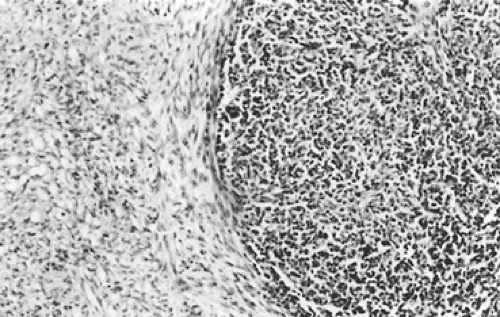
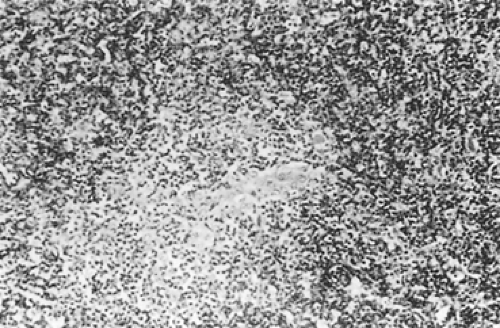
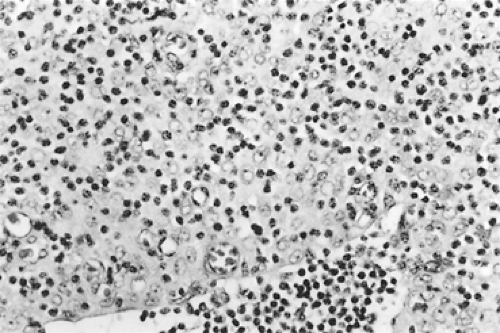
According to Dadmanesh and colleagues,50 type A tumors are composed of oval or spindle-shaped epithelial cells lacking nuclear atypia and accompanied by few or no lymphocytes (Fig. 188-1). The origin of the spindle-shaped cells, considered typical components of the less aggressive thymomas (a medullary variant), is not known. These cells as a rule are not seen in the normal medullary portions of the mature infant or adolescent gland but are seen in small numbers in the periphery of the adult atrophic thymus. According to Rosai,276 these cells correspond to the nonfunctioning thymic epithelial cells that recapitulate spindle cells seen in the involuted thymus of the adult. The tumors are encapsulated, although a few may invade the capsule and even extend into the adjacent fat or lung. Type AB tumors (Fig. 188-2) have foci of type A admixed with foci rich in lymphocytes, as in type B thymomas. Type B tumors resemble the normal functional thymus and are subdivided into types B1, B2, and B3 on the basis of an increasing ratio of epithelial-to-lymphocytic cells and the emergence of atypia of the epithelial cells. Type B1 tumors resemble normal active thymus and are lymphocyte-rich, with some areas resembling the thymic medulla (Fig. 188-3). Type B2 tumors are likewise rich in lymphocytes, but foci of medullary differentiation are less evident or even absent. The epithelial cells are more numerous and are plump cells with vesicular nuclei and conspicuous nucleoli (Fig. 188-4). A palisading appearance may be present owing to the perivascular arrangement of the cells. Type B3 tumors are composed mainly of round or polygonal epithelial cells in a sheet-like growth
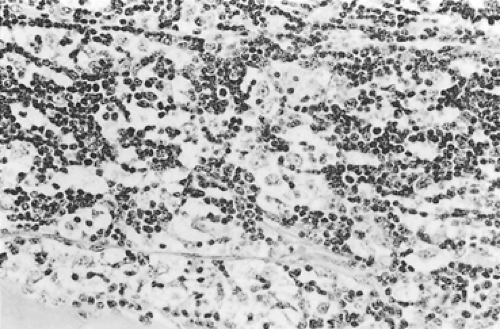
pattern and there is little or no lymphatic component (Fig. 188-5). Atypia may or may not be present. Foci of squamous metaplasia and perivascular spaces are common. Type C tumors are frankly malignant cells; the cytoarchitecture is no longer specific to the thymus. These tumors are discussed in the section on thymic carcinomas. However, as seen in Table 188-4, the WHO classification resembles the Kirchner and Müller-Hermelink classification. The classification of Suster and Moran310,312 is quite different in that the term thymoma (well-differentiated tumors) is used to classify all categories except type B3, which is classified as an atypical thymoma (moderately differentiated). The poorly differentiated tumors are thymic carcinomas. Nevertheless, it must be emphasized that microscopic or gross invasion beyond the capsule of any of the typical (well-differentiated) or type A tumors—including those through B2 and probably also those with atypical or B3 lesions—is probably of more prognostic significance than the histocytologic features. However, it should be noted that although the histology of thymic epithelial tumors has a disputed effect on the patient’s prognosis, Okumura and associates229 were able to show that the incidence of invasive tumor increased with each advancing stage in the WHO classification: (a) type A, 12.5%; (b) type AB, 38.8%; (c) type B1, 40%; (d) type B2, 69.4%; (e) type B3, 80%; and (f) type C, 100%. However, Rosai271 reported that the WHO classification was changed by deleting category C. Travis and associates333 have presented the recent changes in the histologic classification of thymomas by the WHO. The only significant changes were the exclusion of type C thymomas (thymic carcinomas) from the classification, as noted, and the introduction of various unusual subtypes (i.e., micronodular thymoma with B-cell hyperplasia and “metaplastic” thymoma, among other rare variants) that did not fit well in any of the standard categories.
pattern and there is little or no lymphatic component (Fig. 188-5). Atypia may or may not be present. Foci of squamous metaplasia and perivascular spaces are common. Type C tumors are frankly malignant cells; the cytoarchitecture is no longer specific to the thymus. These tumors are discussed in the section on thymic carcinomas. However, as seen in Table 188-4, the WHO classification resembles the Kirchner and Müller-Hermelink classification. The classification of Suster and Moran310,312 is quite different in that the term thymoma (well-differentiated tumors) is used to classify all categories except type B3, which is classified as an atypical thymoma (moderately differentiated). The poorly differentiated tumors are thymic carcinomas. Nevertheless, it must be emphasized that microscopic or gross invasion beyond the capsule of any of the typical (well-differentiated) or type A tumors—including those through B2 and probably also those with atypical or B3 lesions—is probably of more prognostic significance than the histocytologic features. However, it should be noted that although the histology of thymic epithelial tumors has a disputed effect on the patient’s prognosis, Okumura and associates229 were able to show that the incidence of invasive tumor increased with each advancing stage in the WHO classification: (a) type A, 12.5%; (b) type AB, 38.8%; (c) type B1, 40%; (d) type B2, 69.4%; (e) type B3, 80%; and (f) type C, 100%. However, Rosai271 reported that the WHO classification was changed by deleting category C. Travis and associates333 have presented the recent changes in the histologic classification of thymomas by the WHO. The only significant changes were the exclusion of type C thymomas (thymic carcinomas) from the classification, as noted, and the introduction of various unusual subtypes (i.e., micronodular thymoma with B-cell hyperplasia and “metaplastic” thymoma, among other rare variants) that did not fit well in any of the standard categories.
Suter and Moran315 have presented an eloquent discussion of the WHO schema for thymomas (A, AB, B1, B2, and B3). These authors have noted the histologic complexities and at times the contradictions in the classification. They question the assumption that types A and AB behave as “benign” tumors and types B1, B2, and B3 as “malignant” tumors, increasing from low-grade to high-grade lesions: B1 to B3 tumors. On the other hand these authors believe that all thymomas are malignant and believe that the terminology they suggested in 1999322 (Table 188-5) would be more appropriate. It would seem to be prudent to delete their third category, however, since the poorly differentiated thymic carcinomas can be set apart from the thymomas per se. These latter thymic carcinomas histologically do not appear to be composed of thymic elements but of other epithelial cell types (keratinizing squamous cell carcinoma, lymphoepithelioma-like carcinoma, basaloid carcinoma, etc.).
Park and coworkers239 have reported that the “new” WHO histologic classification of thymic tumors is a significant independent prognostic factor with respect to survival, as is the Masaoka stage of the tumor.
However, Detterbeck,61 in a review of a large number of studies of the histologic classification, found that there was marked interobserver discordance in almost every study. In the study conducted by Dawson and associates,54 the various thymomas were assigned to the same group in only 35% of the cases. Rieker and colleagues265 noted that interobserver agreement was only 50% within the B1, B2, and B3 group of thymomas. Of course, different concordance rates have been published. Further indirect evidence illustrating the difficulty in assigning a thymoma to a given cell type are the ranges recorded in a number of studies reviewed by Detterbeck62 (Table 188-6).
For years it has been stressed that thymomas were essentially histologically benign tumors with a malignant potential that increased with the degree of the lack of differentiation of the epithelial cells and the increasing degree of atypia. It is now thought that in fact all thymomas are latent or even actively malignant lesions from their conception, despite their benign histologic appearance; the degree of malignancy being low in the so-called medullary (and the spindle cell) tumors, as well as in the well-differentiated tumors (types A and AB) and high in the epithelial, moderately differentiated tumors (types B1, B2, and B3). The poorly differentiated tumors (thymic carcinomas)
are now separated into their own subgroup, since these tumors have no histologic features of either an adolescent or an atrophic adult thymic gland but reflect the histologic features of many other epithelial carcinomas, as discussed subsequently.
are now separated into their own subgroup, since these tumors have no histologic features of either an adolescent or an atrophic adult thymic gland but reflect the histologic features of many other epithelial carcinomas, as discussed subsequently.
Table 188-4 Comparison of Three Main Histologic Classifications of Thymic Epithelial Neoplasms | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
The new WHO classification published by Travis and associates in 2004330 (see Table 188-5) is used as much as possible in this presentation. However, other classifications will be used of necessity when significant data have been presented under earlier classifications.
Other Macroscopic Features
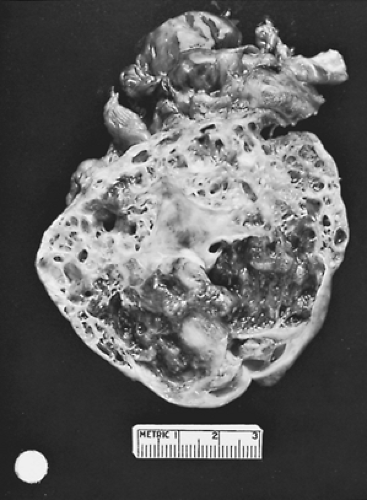
Degenerative changes—especially hemorrhage, calcifications, and cystic changes—are frequently observed in thymic tumors. Rosai and Levine272 reported that microcysts are present in 16% to 40% of tumors, and Gray and Gutowski92 reported that macroscopic ones (i.e., grossly visible cysts) also are seen in as many as 40% of thymomas (Fig. 188-6). These cysts have no relationship to the invasiveness or noninvasiveness of the lesion or to the association of the tumor with the systemic disease syndromes often present with thymomas. According to Moran and Suster,199 such changes (i.e., hemorrhage and necrosis) in well-encapsulated, noninvasive cysts do not portend an adverse prognosis. A grossly cystic thymoma must be differentiated from a true benign thymic cyst.
Microscopic Thymomas (Nodular Hyperplasia) and Microthymomas
The term microscopic thymoma was coined by Rosai and Levine272 to describe tiny, discrete epithelial islands of suggestive abnormal thymic tissue less than 1 cm in size in a thymus removed during a cardiac surgical procedure. Subsequently a few other investigators identified similar islands in thymic glands removed for the treatment of myasthenia gravis (MG). Rosai270 revisited the subject, and although these microscopic islands may resemble a type A thymoma, these lesions are basically of no major importance and deserve little attention. Rosai270 suggested that these small lesions would be more properly defined as nodular hyperplasia within the thymus gland.
However, in regard to small thymic lesions, it should be noted that Cheuk and colleagues41 incidentally identified two small true thymic tumors in two thymic glands that had been removed for the management of red cell aplasia in one patient and the treatment of MG in the second. In the first case the mass was 7 by 4 mm in size and was a typical type B1/B2 thymoma. The second lesion was 5 by 3 mm in size and was readily identified as a type
B2 thymoma. To differentiate such unusual tumors inappropriately labeled as microscopic thymomas, these latter investigators suggested that these tumors be termed microthymomas. A similar tumor had been described in 1997 by Shimosato and Mukai.291 This microthymoma was 5 mm in size and resembled a typical type B2 tumor as defined by the current classification. Whether or not these lesions represent an early “in situ” stage of a true thymoma remains unresolved.
B2 thymoma. To differentiate such unusual tumors inappropriately labeled as microscopic thymomas, these latter investigators suggested that these tumors be termed microthymomas. A similar tumor had been described in 1997 by Shimosato and Mukai.291 This microthymoma was 5 mm in size and resembled a typical type B2 tumor as defined by the current classification. Whether or not these lesions represent an early “in situ” stage of a true thymoma remains unresolved.
Table 188-5 The WHO’s and Suster and Moran’s Terminologies for the Histologic Classification of Thymomas | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Table 188-6 Incidence of Histologic Cell Types in Four Large Series of Thymomas | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
Gross Pathologic Features
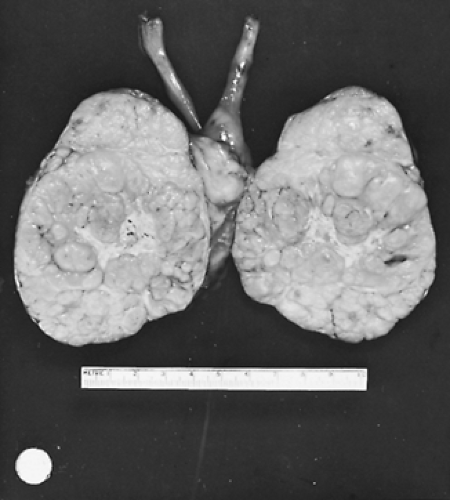
Grossly, a thymoma may be round or oval and may vary greatly in size. Thymomas appear to have a bosselated outer surface but occasionally appear as a flattened mass simulating a fibrotic plaque. The parenchyma is a soft tan or gray-pink fish-flesh–colored tissue with visible lobules and readily apparent white-gray fibrous tissue septa (Fig. 188-7). In almost all instances, the tumor is contiguous with adjacent normal-appearing thymic tissue unless the entire gland has been replaced by the tumor (Fig. 188-8).
Multiple isolated tumors of the thymus gland are very rare; only 14 cases have been recorded. Nonami and Moriki220 reported synchronous bifocal lesions of the same histologic cell type as were the findings in 10 of the other cases that have been recorded. Yoneda and associates374 reported an example of the two tumors being of different histologic cell types and noted a similar case in the literature. Kawaguchi and colleagues130 reported a patient with three separate tumors in the gland. Each cell type was different: a type B2, a type B1, and a type AB thymoma. In the entire group of patients at least one double lesion was thought to be metastatic in origin. In the other 13 patients the multiple thymomas were thought to be multicentric in origin. However, the validity of this assumption remains unknown.
The most important gross features are the presence or absence of encapsulation of the tumor, the presence or absence of fixation to or, more important, gross invasion into adjacent structures, and the presence of pleural implants.
The incidence of well-encapsulated noninvasive “benign” thymomas (many of which would be classified as type A or AB in the 1999 and 2004 WHO classifications) varies in the reported series from 40% to 70%. Bergh21 and Masaoka179 and their associates each reported a 40% incidence; Lewis and colleagues159 reported an incidence of 68%. Occasionally, a well-encapsulated lesion shows microscopic invasion beyond the capsule and thus must be considered an invasive, malignant lesion. Kornstein and associates146 noted 9 of 51 grossly encapsulated lesions (17.6%) to have microscopic invasion through the capsule. They observed four local recurrences in the nine grossly encapsulated lesions that showed microscopic invasion through the capsule into
the adjacent mediastinal fat, as contrasted to no local recurrences in the 42 patients with encapsulated thymomas without microscopic evidence of invasion through the capsule.
the adjacent mediastinal fat, as contrasted to no local recurrences in the 42 patients with encapsulated thymomas without microscopic evidence of invasion through the capsule.
However, although the aforementioned authors, as well as Fujimura76 and Haniuda97 and their associates, reported no recurrence in patients with completely encapsulated thymomas, even the patients with well-encapsulated lesions without microscopic invasion through the capsule may have a small incidence of local recurrence, varying between 2% and 12%, as recorded by Fechner72 and Masaoka,179 Monden,192 and Lewis159 and their colleagues, so that even these noninvasive lesions must be considered to have a malignant potential.
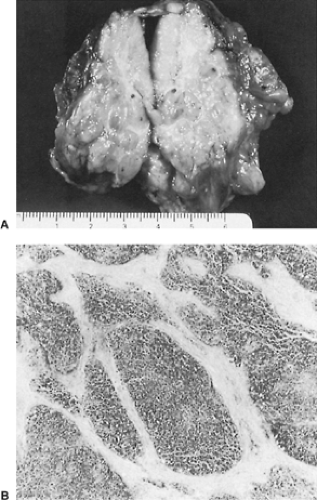
Gross or microscopic invasion of thymomas is present in about 30% to 60% of cases, according to the studies of Verley and Hollmann341 and of Maggi,171 Lewis,159 and Kornstein146 and their associates. When invasion is present, the thymoma must be considered a malignant lesion regardless of the microscopic appearance or cellular structure of the tumor (Fig. 188-9). In fact, even in a malignant thymoma, the cellular structure should be cytologically benign except for occasional atypia of the epithelial cells. The invasion of the tumor into an adjacent structure (e.g., mediastinal pleura, pericardium, lung, lymph nodes, great vessels, nerves, or chest wall) must, however, be documented microscopically to establish the malignant nature of the lesion. A small number of encapsulated lesions may be grossly fixed to one of the aforementioned structures, but invasion is not demonstrated microscopically. In this situation, the lesion must be considered noninvasive. As Lewis and colleagues159 noted, however, the patients with this subgroup of thymoma have a somewhat poorer long-term prognosis than the patients with nonadherent encapsulated tumors. Regnard and coworkers257 observed five local recurrences in the former subgroup but none in the nonadherent subgroup in a long-term follow-up of their patients. On the other hand, the prognosis in this adherent subgroup is generally not as poor as that seen when microscopic invasion is documented.
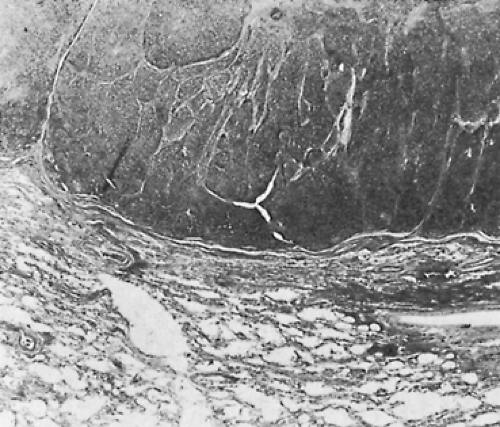
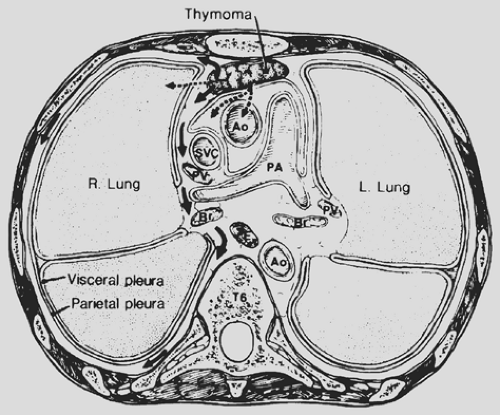
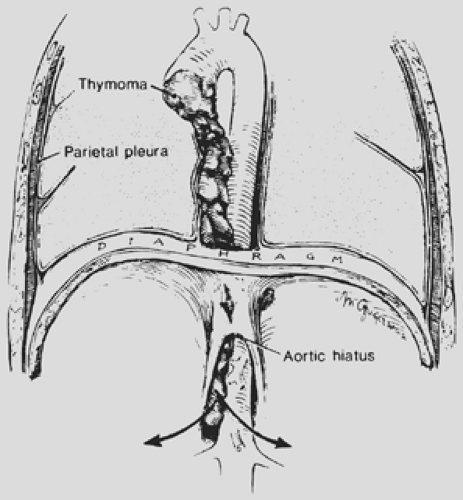
Local invasion may be limited to the most adjacent structures, but extensive spread to more distant sites within the thorax is not uncommon (Fig. 188-10). Those patients with spread to the diaphragm also may have transdiaphragmatic extension. Scatarige and associates285 recorded infradiaphragmatic invasion in 6 of 19 patients (31.5%) with advanced, extensive local disease. The sites included the right lateral liver surface, posterior pararenal space, left para-aortic region, perigastric soft tissues, and spinal canal. These authors suggested that such extension was best identified by upper abdominal computed tomography (CT) examination in those patients with demonstrated diaphragmatic involvement. A number of potential pathways of the subdiaphragmatic extension have been suggested. Zerhouni and colleagues378 note that such extension may occur through the retrocrural space (Fig. 188-11). The communications among the extrapleural space, retrocrural space, and posterior pararenal space have been confirmed by the studies of Borlaza and associates.28 Kleinman and coworkers135 described
a second potential pathway anteriorly through a potential midline defect as well as the two parasternal spaces of Larrey (i.e., the foramen of Morgagni). A third pathway is spread directly through the diaphragm itself.
a second potential pathway anteriorly through a potential midline defect as well as the two parasternal spaces of Larrey (i.e., the foramen of Morgagni). A third pathway is spread directly through the diaphragm itself.
In addition to local invasion, malignant thymomas, despite their benign histologic appearance, may infrequently metastasize to distant sites: pleural or pericardial implants (most often types B2 or B3), the lung, or infrequently to the mediastinal lymph nodes, and extrathoracic sites such as bone, liver, central nervous system, and axillary or supraclavicular lymph nodes. Kondo and Monden143 agreed with the suggestion of Yamakawa and associates365 that the regional lymph nodes of the thymus be classified into three groups: anterior mediastinal lymph nodes (N1), other intrathoracic lymph nodes (N2), and extrathoracic lymph nodes (N3). The aforementioned authors143 recorded that the incidence of lymphogenous metastasis was 1.8% in 1,064 patients with true epithelial thymic tumors. All involved lymph nodes were within the thorax. Wright362 believes that such metastasis should simply be termed intrathoracic lymph node metastasis and that no division into N1 or N2 is necessary or even desirable.
However, it should be noted that 5-year survival was adversely affected by the lymph node involvement. The survival rates were 95.6%, 61.5%, and 20% for N0, N1, and N2 respectively; nonetheless, multivariate analysis demonstrated that survival was dependent on the clinical Masaoka stage and complete resection.
Hematogenous metastasis was likewise infrequent. Only 1.2% of 1,073 patients with thymomas were noted to have hematogenous metastases and 84.6% of them were confined to the lung. Lewis and associates,159 in an earlier review of 283 noninvasive and invasive thymomas seen at the Mayo Clinic, reported an incidence of distant metastasis of 3%. Kornstein146 and Maggi171 and their associates and Verley and Hollmann341 reported that distant metastases occurred in association with invasive tumors in 3%, 5%, and 7% of patients, respectively. In a small series of 36 patients with invasive thymomas, however, Batata and colleagues16 at Sloan-Kettering Memorial Hospital reported an incidence of 30%. Cohen and associates,43 in a similar group of patients with invasive thymomas, reported an incidence of 26%. Blumberg and associates,24 in a later review of the material at Memorial Sloan-Kettering Cancer Center, reported a similar overall distant metastatic rate of 29%. Thus, it is apparent that patient referral patterns are important in any evaluation of the data presented.
Staging of Thymomas
Because of the biologic variability of the epithelial cell tumors of the thymus, a number of classifications have been suggested to stage these lesions relative to their invasiveness, metastatic characteristics, and the potential prognosis of the various substages. The staging schemes of Bergh21 and Masaoka179 and their colleagues, as well as that of Suster and Moran,315 are a few of the many suggested schemes, but at present the scheme suggested by Masaoka and coworkers179 is the most commonly used (Table 188-7). However, the modifications of Masaoka and associates’ scheme suggested by Trastek and Payne332 and that of Kornstein145 would also appear to be appropriate for general use (Table 188-8).
Haniuda and colleagues97 proposed the addition of the status of mediastinal pleural involvement (no adhesion to the tumor, P0; fixation but not invasion, P1; and microscopic invasion of
the mediastinal pleura, P2) because of the greater likelihood of local recurrence with each advancing degree of involvement (P1 versus P0 and P2 versus P1).
the mediastinal pleura, P2) because of the greater likelihood of local recurrence with each advancing degree of involvement (P1 versus P0 and P2 versus P1).
Table 188-7 Staging Schemes of Thymoma | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Moreover, the French surgeons Verley and Holliman,341 as well as Gamondès86 and Regnard257 and their associates, have suggested the inclusion of the extent of resection, complete or incomplete, because the latter significantly lowered the survival rate in stage III disease. These schemata were variations of the staging of the Group d’Etudes des Tameurs Thymiques (French GETT classification, 1982) published by Gamondès and colleagues in 199186 (Table 188-9). Of these schemata, that of Regnard and associates257 is the most complete and is more similar to the Masaoka scheme. Nonetheless, although the P factor and the completeness of resection should be considered in assigning a tentative prognosis in a given patient, neither needs to be incorporated into the basic staging system. Recently, Asamura and coworkers11 suggested a new staging system by combining stage I and stage II disease as stage I, with capsular invasion being disregarded as a staging criterion. New stage II or III was then determined by size of the tumor: ≤10 cm or ≥10 cm in diameter, and whether one or more intrathoracic organs were involved. Stage IV remained the same. At the moment, the acceptance of this schema is questionable. Yamakawa and colleagues365 attempted to develop a tumor, node, metastasis (TNM) classification but essentially found it of no value for the staging of thymomas. Recently Bedini and associates13 likewise have proposed a TNM-based staging system. However, since thymic carcinomas (C) are no longer included in the classification of thymomas, the data used to support their proposal are not relative. This is mainly due to the near complete absence of lymph node and
distant metastases, 1.8% and 1.2% respectively, associated with true thymomas. However, in patients with thymic carcinomas, the incidence is 26.8% and 12% respectively, as recorded by Kondo and Monden.143
distant metastases, 1.8% and 1.2% respectively, associated with true thymomas. However, in patients with thymic carcinomas, the incidence is 26.8% and 12% respectively, as recorded by Kondo and Monden.143
Table 188-8 Suggested Simplified Staging of Thymomas | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Immunohistochemical Studies
At present, immunohistochemical studies of thymomas are primarily used in the identification of thymic epithelial cells to distinguish thymoma from lymphoma or other malignant anterior mediastinal tumors. According to Kornstein and colleagues,146 antibodies to cytokeratin are most useful. Also, the finding that the lesion is negative for chromogranin provides a basis for the distinction between thymic carcinoids (positive for chromogranin) and a true epithelial thymoma (negative for chromogranin). The immunohistochemistry of thymomas is presented in Table 188-10. Pan and colleagues235 have presented data that B lymphocyte–associated CD20 antigen is present in the epithelial cells (spindle cells) in most type A and type AB thymomas, whereas the epithelial cells in type B1 to B3 thymomas did not express this antigen. The explanation for these differences remains to be elucidated.
Tomita and colleagues330 have reported that there may be an association between tumor angiogenesis (and the expression of vascular endothelial growth factor [VEGF]) and the invasiveness of thymomas. In immunohistochemical studies of 38 resected tumors (18 noninvasive thymomas and 20 invasive thymomas), the mean microvessel densities were 4.6 ± 3.2 and 12.4 ± 7.5, respectively. VEGF expression was present in 5.6% and 55.0%, respectively, in the two aforementioned tumor subsets. From these data, the authors suggested that there was a significant correlation between tumor angiogenesis and invasiveness. Additional studies on tumors separated into the subgroups of the WHO classification are necessary to define the importance of the presence of VEGF expression and tumor angiogenesis.
Table 188-10 Immunohistochemistry of Epithelial Thymomas | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Clinical Presentation
Most patients with thymomas are adults in the fifth and sixth decades of life, although thymomas may occur at any age. A thymoma is rarely discovered in children. Whittaker and Lynn348 reported no cases in 105 children with mediastinal lesions seen at the Mayo Clinic. Isolated case reports have appeared in the literature since their report, however, and these cases were reviewed by Furman and associates.83 La Franchi and Fonkalsrud152 reported four benign and three malignant thymomas in children <20 years of age; the youngest was 4 and the oldest 19. Welch and associates347 reported five malignant lesions, only three of which meet the present-day criteria of an epithelial thymoma; only two patients were <16 years of age. In Lewis and colleagues’159 series from the Mayo Clinic, no patients with a thymoma were <16 years of age. However, Ramon y Cajal and Suster253 and Pescarmona and associates,246 respectively, recorded 10 and 5 typical lymphocytic thymomas in children.
In adults, the sex distribution is about equal between men and women. In the various series reported, there may be a slight preponderance of one gender or the other, but no significant difference occurs in the overall numbers.
Patients with thymomas are often clinically asymptomatic. The exact percentage is difficult to ascertain, although 50% is often quoted. The symptomatic patients may have only local symptoms related to the presence of the tumor within the mediastinum, only symptoms related to the systemic disease states that are frequently associated with the presence of a thymoma, or a combination of both. As a consequence of this, as well as of the marked differences in the classification of thymic tumors in the past, it is impossible to state with assurance the number of truly symptomatic patients. In most reviews in the literature, about 30% to 40% of patients have local symptoms, and 30% to 50% have an associated systemic parathymic disease syndrome. Overlap of the two categories of symptoms occurs frequently but is not often separated in the available reviews. In Detterbeck and Parsons’62 review, the overall data are not markedly dissimilar to the aforementioned percentages.
Local Symptoms and Signs
As noted, probably 50% to 60% of patients are asymptomatic relative to the presence of the thymoma within the chest. When there are local symptoms, however, vague chest pain, shortness of breath, and cough are the common complaints. Severe chest pain, superior vena cava obstruction, paralysis of a hemidiaphragm as a result of involvement of a phrenic nerve, and hoarseness caused by involvement of a recurrent laryngeal nerve are infrequent but ominous signs of extensive malignant disease. The presence of a pleural or pericardial effusion is likewise a serious clinical finding. On rare occasion, a thymoma will spontaneously rupture; the resulting acute mediastinal hemorrhage is associated with severe chest pain and dyspnea. Radiographs will reveal mediastinal widening due to the hemothorax present. Appropriate radiographic studies will rule out a major vascular tear. Stabilization of the patient and subsequent thymectomy are indicated. Only a few cases have been recorded in the literature, such as those reported by Caplin,33 Templeton,325 Fukuse,81 and Shimokawa292 and their colleagues. dePerrot and associates61 recorded a case, as did Wright and Wain,360 who reported one patient with hemorrhage without rupture but associated with infarction of the gland. Santoprete and colleagues282 recorded a massive hemorrhage induced by a spontaneous rupture of a giant thymoma. Infarction of a thymoma is likewise uncommon. Carr and O’Keefe34 reported the spontaneous infarction of an ectopically located thymoma, but rupture of the resulting cyst did not occur. In addition to this latter case, Kuo,151 Gray and Windsor,93 and Wright and Wain360 have presented additional cases of infarction of a thymoma. The latter authors suggest complete excision of the affected gland. They report a satisfactory prognosis for patients with this approach.
Infection occurring in a thymoma is rare. Carter and associates36 recorded a Salmonella infection in a cyst within a type B thymoma that was successfully treated by percutaneous drainage and subsequent complete thymectomy.
Lewis and associates159 reported the presence of weight loss, fatigue, fever, night sweats, and other constitutional symptoms in 18% of their patients with thymoma. The clinical significance of these constitutional findings is difficult to determine.
Associated Systemic (Parathymic) Disease States
A thymoma is rare; thus its coincidence with other conditions is noteworthy and eventually may lead to further understanding of thymic function in health and disease. In Rosenow and Hurley’s279 review, 40% of patients with thymoma had a parathymic syndrome, and one-third of this group had two or more parathymic syndromes. Table 188-11 lists the clinical disorders associated with thymomas, and Table 188-12 lists the varying incidences of the more common parathymic syndromes that were published by Verley and Hollmann341 as well as by Souadjian304 and Lewis159 and their colleagues.
Myasthenia Gravis
MG is the most commonly associated disease. This syndrome is present in about 30% of patients with thymomas. Higher incidences have been reported by Lewis,159 Maggi,171 and Monden191 and their associates, 46%, 73%, and 59%, respectively. These high incidences are undoubtedly the result of specific patient referral patterns. These patients tend to be, on average, 10 to 15 years older than patients with myasthenia without tumor and somewhat younger than patients who have thymoma without this complication. Patients with thymoma and MG may have any type of thymoma except that its occurrence with the type A thymoma (spindle cell, medullary) variety is rare, as noted by Verley and Hollmann,341 Lewis,159 and Maggi171,172 and their colleagues. Kirchner and associates132 reported a marked association of squamous elements in thymomas with MG. Kornstein145 could not confirm this finding. Although the association of myasthenia with thymoma is usually simultaneous, thymomas have been recognized years after the onset of myasthenia;
conversely, myasthenia has developed promptly or even years after the removal of a thymic neoplasm, as noted by Namba and colleagues215 in their report in 1978. Onoda and associates231 described the onset of fulminant MG after the removal of a misdiagnosed thymoma. Medical therapy as well as subsequent completion thymectomy was necessary to control the patient’s disease. Mulder,204 in an invited commentary, suggested that at the time of removal of any unknown anterior mediastinal mass, an immediate histologic diagnosis be obtained so that a total thymectomy can be done if the tumor indeed is a thymoma, because the onset of postoperative MG, although uncommon, is not rare. Namba and colleagues215 have discussed the vagaries of this occurrence. Overall, most studies have reported an incidence of approximately 3% of postresection MG after resection of thymomas. These cases occurred as early as 2 weeks to as late as 72 months after the resection. Ito and colleagues121 recorded 13 (3.3%) cases after 389 resections of thymomas. However, Kondo and Monden139 reported an incidence of only 0.97% (8 cases in 827 patients) after resection of a thymoma not associated with a preoperative myasthenic state. The occurrence of this complication is slightly more common in women. At present more than 80% of these patients respond to anticholinesterase compounds and/or steroids. The prognosis is considered to be good. Of interest is the report of Fang and colleagues71 from Shanghai, China, who reviewed 204 thymic epithelial tumors. Most of their data support the current concepts discussed previously. However, early in their experience the authors had high morbidity and mortality rates in patients with associated MG; the mortality rate was 50%. In 1986 they began to use preoperative steroid therapy in those patients with thymoma and associated MG. The morbidity rate decreased and the mortality rate in these patients was reduced to zero since the adoption of this preoperative therapy. The report by Endo and coworkers69 of the perioperative use of steroids in nonthymomatous MG patients supports the aforementioned authors’ use of steroid therapy in those patients with both a thymoma and MG. Sekine and coauthors287 agree that alternate-day administration of high-dose prednisolone for a 6-month period prior to thymectomy for the treatment of generalized MG markedly reduces the incidence of postoperative myasthenic crisis. However, some deleterious side effects—such as cushingoid symptoms, steroid-induced diabetes, osteoporosis, and cataract formation—may occur during the treatment period.
conversely, myasthenia has developed promptly or even years after the removal of a thymic neoplasm, as noted by Namba and colleagues215 in their report in 1978. Onoda and associates231 described the onset of fulminant MG after the removal of a misdiagnosed thymoma. Medical therapy as well as subsequent completion thymectomy was necessary to control the patient’s disease. Mulder,204 in an invited commentary, suggested that at the time of removal of any unknown anterior mediastinal mass, an immediate histologic diagnosis be obtained so that a total thymectomy can be done if the tumor indeed is a thymoma, because the onset of postoperative MG, although uncommon, is not rare. Namba and colleagues215 have discussed the vagaries of this occurrence. Overall, most studies have reported an incidence of approximately 3% of postresection MG after resection of thymomas. These cases occurred as early as 2 weeks to as late as 72 months after the resection. Ito and colleagues121 recorded 13 (3.3%) cases after 389 resections of thymomas. However, Kondo and Monden139 reported an incidence of only 0.97% (8 cases in 827 patients) after resection of a thymoma not associated with a preoperative myasthenic state. The occurrence of this complication is slightly more common in women. At present more than 80% of these patients respond to anticholinesterase compounds and/or steroids. The prognosis is considered to be good. Of interest is the report of Fang and colleagues71 from Shanghai, China, who reviewed 204 thymic epithelial tumors. Most of their data support the current concepts discussed previously. However, early in their experience the authors had high morbidity and mortality rates in patients with associated MG; the mortality rate was 50%. In 1986 they began to use preoperative steroid therapy in those patients with thymoma and associated MG. The morbidity rate decreased and the mortality rate in these patients was reduced to zero since the adoption of this preoperative therapy. The report by Endo and coworkers69 of the perioperative use of steroids in nonthymomatous MG patients supports the aforementioned authors’ use of steroid therapy in those patients with both a thymoma and MG. Sekine and coauthors287 agree that alternate-day administration of high-dose prednisolone for a 6-month period prior to thymectomy for the treatment of generalized MG markedly reduces the incidence of postoperative myasthenic crisis. However, some deleterious side effects—such as cushingoid symptoms, steroid-induced diabetes, osteoporosis, and cataract formation—may occur during the treatment period.
Table 188-11 Clinical Disorders Associated with Thymomas | ||
|---|---|---|
|
Table 188-12 Incidence of Conditions Associated with Thymomas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the reviews of Lewis,159 Maggi,171,172 Pescarmona,245 Crucitti,48 Lardinois,55 Okumura,229 and de Perrot58 and their colleagues as well as the report of Kondo and Monden,141 the presence or absence of MG has little overall effect on either the local presentation, clinical behavior, or prognosis of a thymoma. Monden and associates,191,192 however, reported data suggesting that a nonmyasthenic thymoma behaves in a more malignant fashion than a myasthenic thymoma. Elert and colleagues68 noted that patients with MG and a thymoma presented at an earlier stage (only 19% of these patients had an advanced stage of the tumor and had a poorer prognostic outlook) and had a better prognosis than those with a thymoma without MG. Moore and associates193 have made a similar observation. Kondo and Monden,141 in a review of 1,089 patients with a thymoma, found that the presence of associated MG conferred either no effect or even a salutary one on the thymoma’s malignant behavior. The prognosis was the same for both the non-MG patients and those with this associated disease in Masaoka stages I, II, and III, but it was improved in stage IV. The reasons for this latter phenomenon remain conjectural. These observations need further clinical documentation.
Okumura and colleagues229 noted that myasthenia was not seen with type A but occurred in patients with the B varieties (Table 188-13). However, Pan and associates235 have seen several patients with type A thymomas and MG. Earlier, Hirokawa and colleagues107 reported that all 10 of the thymomas
associated with MG in their series of 45 thymomas were positive by immunohistochemical techniques for UH-1 and Leu-7. As a consequence, they considered all of these thymomas to be of cortical epithelial cell origin.
associated with MG in their series of 45 thymomas were positive by immunohistochemical techniques for UH-1 and Leu-7. As a consequence, they considered all of these thymomas to be of cortical epithelial cell origin.
Table 188-13 Myasthenia Gravis Tumor Type and Number of CD4+/CD8+ Lymphocytes | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
In contrast to the high incidence of MG in patients with thymoma, only 5% to 15% of patients with MG have thymomas.
Pure Red Cell Aplasia
The association of thymoma with severe anemia caused by suppression of erythrogenesis in the bone marrow is well established. Whereas red cell precursors are greatly reduced or absent, myelopoiesis and megakaryocytopoiesis are usually normal or increased. Burrows and Carroll,31 however, reported the occurrence of pancytopenia. The mechanism of this erythrocyte aplasia is not clear, but evidence put forth by Jepson and Vas123 suggests that it is mediated immunologically. The presence of immunoglobulin G antibodies—which inhibit erythropoietin or hemoglobin synthesis or both and are cytotoxic for erythroblasts—has been observed in these patients. The immunodeficiency is generally attributed to a decreased number of B lymphocytes in the bone marrow and peripheral blood, as noted by Cooper and Butler.45 The T cells are usually normal in number, although some patients have changes in cellular immunity. Of 56 patients with red cell aplasia reviewed by Hirst and Robertson,108 7 also had MG, and 2 had hypogammaglobulinemia. Of interest was the report of Suzuki and colleagues317 on the occurrence of both pure red cell aplasia and MG after resection of an invasive thymoma. In a recent review by Murakawa and colleagues210 of 166 Japanese patients with thymomas, the incidence of pure red cell aplasia was 3.6% (6 cases), whereas the incidence of MG was 36.7% (61 cases). The combination of the two diseases occurred only once, as did the occurrence of hypogammaglobulinemia. In both instances the second disease occurred after the thymectomy. In three of the patients in the study, pure red cell aplasia developed as a complication after resection of the thymoma, two within 1 year and the third in the eighth year, respectively.
Fifty percent of patients with red cell aplasia have associated thymomas, but only 5% of patients with thymoma have the disorder. According to Beard and colleagues,18 most of these patients (about 70%) have a noninvasive spindle cell variant of thymoma (type A).
Although Zeok and associates377 reported that about 25% to 33% of patients with red cell aplasia benefit from excision of the thymoma and that most, as noted, are associated with type A (the spindle cell subtype) thymoma, which normally has a good prognosis, this group of patients as a whole has a poorer prognostic outlook than those without this autoimmune disease. Kaiser and Martini125 as well as Masaoka,180 Maggi,172 and Murakawa210 and their colleagues have documented this observation. Four of the patients in the latter study died as the result of the disease within 7 to 144 months after the thymectomy.
Neurogenic Syndromes
Neuromyotonia.
An associated neuromuscular disorder, neuromyotonia (neuromuscular hyperactivity syndrome) is infrequently seen in patients with a thymoma. MG may or may not be present. When this combination occurs, the neuromyotonia may predate or postdate the resection of the thymoma. Nicholas and colleagues216 identified seven of these cases in the literature. These patients were reported by McComas184 and Litchy and Auger162 as well as by Halbach,96 Garcia-Merino,87 and Perini240 and their associates. However Halbach and colleagues96 believed that their two cases were distinct from neuromyotonia because of other features present in the two patients. The latter authors placed their patients in the syndrome referred to as chroée fibrillaire de Morvan. This syndrome was described by Morvan in 1890203 in France, and numerous other studies of the syndrome were published during the twentieth century.55 One of interest was the report of Laterre and coworkers,154 who described a case associated with a thymoma.
Stiff-Man Syndrome.
Moersch and Woltman189 described a rare neurologic disorder associated with muscular rigidity and cramps. The cause is unknown but the syndrome, which they named the “stiff-man” syndrome, is believed to be an autoimmune disorder. It may be associated with other autoimmune diseases and at times it may be a paraneoplastic phenomenon. There have been six reported cases associated with a thymoma (Table 188-14); three were of type B1 or B2, one was a type AB, and the type was not noted in the remaining two. The ages ranged from 40 to 79 years and the gender distribution was equal. Of more interest was the marked elevation of the serum titer of antiglutamic acid decarboxylase (GAD) antibodies in the three patients in whom the GAD serum titer was determined. It is thought that an increase in the titer of this antibody interferes with the orderly synthesis of gamma-aminobutyric acid, an important suppressive neurotransmitter in the central nervous system, and that this process may be involved in the occurrence of the syndrome. Following a thymectomy in five of the patients, all but one did well. The symptoms resolved and the serum titer of GAD returned to normal. In the elderly woman who did not respond well, the titer initially decreased but soon became elevated and the symptoms recurred. She was treated with multiple plasmaphereses and the use of baclofen and chonidine and eventually became asymptomatic. A summary of the data relative to these six patients is presented in Table 188-14.
Immunoglobulin Deficiency
The association of thymoma, particularly type A (the spindle cell type of thymoma), and acquired hypogammaglobulinemia, first reported by Good,91 has been described in about 10% of
patients with an immunoglobulin deficiency. The syndrome may occur in a previously normal patient after the removal of a thymoma. Waldmann and coworkers344 reported that this acquired hypogammaglobulinemia occurs mainly in elderly patients. These authors demonstrated a population of suppressor T cells inhibiting immunoglobulin synthesis in several patients with thymoma, although most patients with the syndrome have normal numbers of circulating T cells, normal results of in vitro immunologic tests, and normal skin reactivity to common antigens. Removal of the thymoma is not beneficial in ameliorating the syndrome. The prognosis for these patients is poor.
patients with an immunoglobulin deficiency. The syndrome may occur in a previously normal patient after the removal of a thymoma. Waldmann and coworkers344 reported that this acquired hypogammaglobulinemia occurs mainly in elderly patients. These authors demonstrated a population of suppressor T cells inhibiting immunoglobulin synthesis in several patients with thymoma, although most patients with the syndrome have normal numbers of circulating T cells, normal results of in vitro immunologic tests, and normal skin reactivity to common antigens. Removal of the thymoma is not beneficial in ameliorating the syndrome. The prognosis for these patients is poor.
Table 188-14 Stiff-Man Syndrome Associated with a Thymoma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree