The aim of the present study was to assess, using 3-dimensioanl echocardiography, the morphologic characteristics, determinants, and physiologic limits of left ventricular (LV) remodeling in 511 Olympic athletes (categorized in skill, power, mixed, and endurance sport disciplines) and 159 sedentary controls matched for age and gender. All subjects underwent 3-dimensional echocardiography for the assessment of LV volumes, ejection fraction, mass, remodeling index (LV mass/LV end-diastolic volume), and systolic dyssynchrony index (obtained by the dispersion of the time to minimum systolic volume in 16 segments). Athletes had higher LV end-diastolic volumes (157 ± 35 vs 111 ± 26 ml, p <0.001) and mass (156 ± 38 vs 111 ± 25 g, p <0.001) compared to controls. Body surface area and age had significant associations with LV end-diastolic volume (R 2 = 0.49, p <0.001) and mass (R 2 = 0.51, p <0.001). Covariance analysis showed that also gender and type of sport were significant determinants of LV remodeling; in particular, the highest impact on LV end-diastolic volume and mass was associated with male gender and endurance disciplines (p <0.001). Regardless of the type of sport, athletes had similar LV remodeling indexes to controls (1.00 ± 0.06 vs 1.01 ± 0.07 g/mL, p = 0.410). No differences were found between athletes and controls for the ejection fraction (62 ± 5% and 62 ± 5%, p = 0.746) and systolic dyssynchrony index (1.06 ± 0.40% and 1.37 ± 0.41%, p = 0.058). In conclusion, 3-dimensional echocardiographic morphologic and functional assessment of the left ventricle in Olympic athletes demonstrated a balanced adaptation of LV volume and mass, with preserved systolic function, regardless of specific disciplines participated.
Morphologic left ventricular (LV) changes associated with long-term, intensive exercise training have been extensively reported. Previous echocardiographic surveys have described the upper physiologic limits of LV chamber enlargement and wall thickening in athletes in relation to gender, race, and sport discipline. However, most of these studies were based on linear dimensions derived from M-mode and 2-dimensional echocardiography and were unable to describe the complex 3-dimensional geometry of the left ventricle. Recently, real-time 3-dimensional echocardiography (3DE) has proved reliable to accurately quantify LV volumes and mass, in a fashion similar to cardiac magnetic resonance, in a broad spectrum of populations, including competitive athletes. The aim of the present study was to assess, using 3DE, the morphologic characteristics, determinants, physiologic limits, and functional properties of LV remodeling in a large population of highly trained Olympic athletes involved in different sport disciplines.
Methods
From January to July 2008 and from April to June 2009, we evaluated 515 consecutive Olympic athletes, members of the Italian national team, selected for participation in the 2008 Beijing Olympic Games and the 2009 Pescara Pan-Mediterranean Games. All athletes were evaluated at the Institute of Sports Medicine and Science during period of intensive training.
According to our protocol, each athlete underwent physical examination, rest and exercise 12-lead electrocardiography, and 2-dimensional and 3-dimensional and Doppler echocardiographic examinations. After clinical evaluation, 4 athletes were excluded for evidence of cardiac abnormalities (blood pressure consistently >140/90 mm Hg [n = 2], mitral valve prolapse with regurgitation [n = 1], bicuspid aortic valve with regurgitation [n = 1]); therefore, the final study population comprised 511 athletes.
The mean age was 26 ± 5 years (range 15 to 45), and 363 athletes were male (71%). We arbitrary classified the athletes into 4 subgroups in relation to the predominant characteristics of training: (1) skill (i.e., primarily technical activities [n = 72]), including golf, table tennis, equestrian, artistic gymnastic, shooting, fencing, karate, taekwondo, and sailing; (2) power activities (i.e., primarily isometric activities [n = 70]) including weightlifting, wrestling, and short-distance running (100 to 200 m); (3) mixed disciplines (i.e., disciplines with isometric and isotonic components [n = 136]), including soccer, basketball, volleyball, handball, water polo, and tennis; and (4) endurance disciplines (i.e., primarily isotonic activities [n = 233]), including rowing and canoeing, swimming, long-distance running and marathon, cycling, triathlon, and pentathlon.
All athletes had competed at the national level for ≥3 years (mean 11 ± 5 years). Twenty-two were medalists at the 2008 Beijing Olympic Games and 96 at the 2009 Pescara Pan-Mediterranean Games.
A group of 159 healthy volunteers matched for age (mean 27 ± 4 years, range 18 to 40) and gender (67% men) were also enrolled in this study; they were either completely sedentary or engaged in <3 hours of exercise practice per week. None was involved in sports competitions. Untrained controls underwent physical examinations, baseline electrocardiography, and 3DE.
All subjects agreed to take part in the study, and the protocol was approved by the institutional review board. This study was supported by the Italian National Olympic Committee.
Echocardiographic examinations were obtained by using an iE33 system (Philips Medical Systems, Andover, Massachusetts) equipped with an S3 probe (2 to 4 MHz) for 2-dimensional and Doppler measurements and with an X3-1 matrix-array transducer (1.9 to 3.8 MHz) for 3-dimensional examinations. Two-dimensional assessment of LV cavity diameters, wall thickness, and left atrial and aortic root diameter were performed according to American Society of Echocardiography criteria.
Three-dimensional echocardiographic acquisitions were performed from the apical 4-chamber view using the full-volume technique with high frame rate priority (31 ± 4 Hz).
LV volumes and mass were analyzed using commercially available software (QLAB version 5.0; Philips Medical Systems), as previously described. The systolic dyssynchrony index (SDI) was calculated as the standard deviation of the time to minimum systolic volume in 16 LV segments, normalized by the RR interval. The LV remodeling index (LVRI) was calculated as the ratio of LV mass to LV end-diastolic volume.
Calculation of LV volumes was feasible in all subjects; LV mass assessment was not achievable in 23 subjects (3.4%) because of artifacts, suboptimal definition of the epicardial border, or LV size exceeding the pyramidal ultrasound beam.
Standard 12-lead electrocardiography was performed with the subject in the supine position and recorded at 10 mV and 25 mm/s. Abnormal electrocardiographic findings were defined as the presence of ≥1 of the following criteria (suggesting the presence of possible cardiomyopathy): inverted T waves of ≥2 mm in ≥2 leads, Q waves ≥4 mm in depth and present in ≥2 leads, left or right bundle branch block, and marked left (≤−30°) or right (≥110°) QRS axis deviation.
According to our protocol, athletes with clinical histories of palpitations or syncope and/or ≥3 premature ventricular beats at the baseline or exercise test underwent 24-hour Holter monitoring.
Continuous data are expressed as mean ± SD. Categorical data are expressed as frequencies. Differences between proportions were calculated using the chi-square test. Statistical significance was set for a 2-tailed p value <0.05.
One-way analysis of variance with multiple Bonferroni post hoc tests was used to assess differences between untrained controls and athletes according to the types of sports in which they participated. Pearson’s simple correlation was performed to test the relation between LV end-diastolic volume and LV mass.
Stepwise regression analysis was used to assess the impact that several variables (age, body surface area [BSA], and systolic and diastolic blood pressure) had on LV end-diastolic volume and mass; only continuous variables significantly correlated with LV end-diastolic volumes and mass were subsequently incorporated in the covariance analysis, together with gender and type of sport (as categorical variables). These 2 categorical variables were coded using a series of n −1 binary dummy variables, and their impact (i.e., regression coefficients) on LV end-diastolic volume and mass was assessed after removing the effect of continuous covariates.
Specifically, to evaluate the relative impact of type of sport and gender, we selected untrained controls as the reference group and female as the reference gender. We therefore calculated the regression coefficients for each type of sport and gender as previously described. The significance of each variable in the covariance analysis was assessed using the Wald test.
To assess reproducibility, measurements of LV volumes and mass were repeated in a random sample of 30 subjects by the first investigator ≥1 week after the previous measurement (intraobserver variability) and by a second investigator (interobserver variability). Investigators were blinded to each other’s results and to patient data. Inter- and intraobserver variability was calculated as the difference between the 2 measurements in terms of the percentage of their mean. Statistical analysis was performed using SPSS software version 15.0 (SPSS, Inc., Chicago, Illinois).
Results
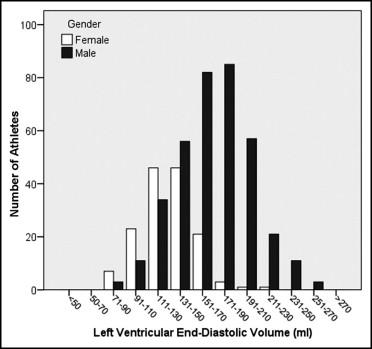
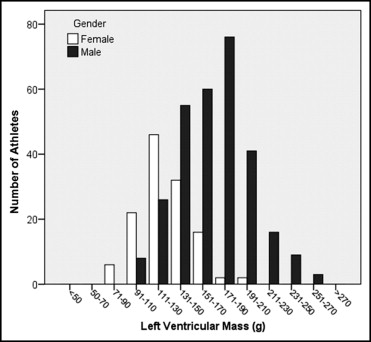
Demographic characteristics of athletes and untrained controls are listed in Table 1 . The distribution of LV end-diastolic volume and mass among male and female athletes is displayed Figures 1 and 2 . The means and ranges of values in healthy volunteers and athletes according to sport disciplines are listed in Table 2 for men and Table 3 for women.
| Variable | Controls (n = 159) | Skill (n = 72) | Power (n = 70) | Mixed (n = 136) | Endurance (n = 233) |
|---|---|---|---|---|---|
| Male | 106 (67%) | 50 (69%) | 48 (69%) | 97 (71%) | 168 (72%) |
| Age (years) | 27 ± 4 | 26 ± 6 | 26 ± 4 | 27 ± 7 | 25 ± 5 |
| BSA (m 2 ) | 1.75 ± 0.20 ‡ , § , ∥ | 1.83 ± 0.19 § , ∥ | 1.89 ± 0.22 ⁎ , § | 2.03 ± 0.19 ⁎ , † , ‡ , ∥ | 1.92 ± 0.21 ⁎ , † , § |
| Systolic blood pressure (mm Hg) | 118 ± 15 | 115 ± 12 | 116 ± 10 | 119 ± 9 | 118 ± 10 |
| Diastolic blood pressure (mm Hg) | 74 ± 8 | 75 ± 7 | 76 ± 6 | 77 ± 6 | 75 ± 6 |
| Heart rate (beats/min) | 76 ± 15 † , ‡ , § , ∥ | 59 ± 12 ⁎ , ∥ | 58 ± 12 ⁎ | 56 ± 9 ⁎ | 53 ± 10 ⁎ , † |
| Variable | Controls (n = 106) | Skill (n = 50) | Power (n = 48) | Mixed (n = 97) | Endurance (n = 168) |
|---|---|---|---|---|---|
| LV end-diastolic volume (ml) | 121 ± 24 † , ‡ , § , ∥ | 138 ± 29 ⁎ , § , ∥ | 150 ± 27 ⁎ , ∥ | 164 ± 29 ⁎ , † , ∥ | 186 ± 28 ⁎ , † , ‡ , § |
| (73–191) | (89–221) | (90–209) | (109–240) | (119–268) | |
| LV end-diastolic volume index (ml/m 2 ) | 64 ± 11 † , ‡ , § , ∥ | 73 ± 11 ⁎ , ∥ | 77 ± 11 ⁎ , ∥ | 78 ± 12 ⁎ , ∥ | 93 ± 12 ⁎ , † , ‡ , § |
| (41–100) | (49–102) | (55–98) | (53–113) | (56–136) | |
| LV ejection fraction (%) | 62 ± 5 | 64 ± 6 | 63 ± 6 | 64 ± 5 | 64 ± 5 |
| (51–71) | (53–71) | (50–73) | (51–71) | (50–78) | |
| LV mass (g) | 123 ± 22 ‡ , § , ∥ | 152 ± 27 § , ∥ | 152 ± 27 ⁎ , ∥ | 169 ± 28 ⁎ , † , ∥ | 185 ± 28 ⁎ , † , ‡ , § |
| (67–175) | (93–197) | (98–237) | (118–245) | (116–266) | |
| LV mass index (g/m 2 ) | 65 ± 11 ‡ , § , ∥ | 72 ± 10 § , ∥ | 81 ± 11 ⁎ , ∥ | 81 ± 11 ⁎ , † , ∥ | 93 ± 13 ⁎ , † , ‡ , § |
| (38–94) | (51–91) | (56–104) | (53–112) | (60–134) | |
| LVRI (g/ml) | 1.01 ± 0.08 | 1.01 ± 0.06 | 1.03 ± 0.09 | 1.01 ± 0.06 | 1.01 ± 0.07 |
| (0.84–1.20) | (0.84–1.10) | (0.83–1.32) | (0.84–1.18) | (0.78–1.28) | |
| SDI (%) | 1.37 ± 0.41 | 1.15 ± 0.43 | 0.94 ± 0.26 | 1.05 ± 0.41 | 1.05 ± 0.41 |
| (0.66–2.43) | (0.42–2.27) | (0.46–1.47) | (0.27–2.49) | (0.26–2.87) |
| Variable | Controls (n = 53) | Skill (n = 22) | Power (n = 22) | Mixed (n = 39) | Endurance (n = 65) |
|---|---|---|---|---|---|
| LV end-diastolic volume (ml) | 90 ± 14 † , ‡ , § , ∥ | 110 ± 19 ⁎ , § , ∥ | 112 ± 20 ⁎ , § , ∥ | 144 ± 22 ⁎ , † , ‡ | 133 ± 17 ⁎ , † , ‡ |
| (52–126) | (87–158) | (74–148) | (103–215) | (101–175) | |
| LV end-diastolic volume index (ml/m 2 ) | 55 ± 7 † , ‡ , § , ∥ | 66 ± 9 ⁎ , § , ∥ | 63 ± 10 ⁎ , § , ∥ | 77 ± 10 ⁎ , † , ‡ | 78 ± 9 ⁎ , † , ‡ |
| (34–72) | (53–89) | (51–87) | (62–107) | (58–99) | |
| LV ejection fraction (%) | 64 ± 4 | 65 ± 5 | 64 ± 6 | 65 ± 5 | 63 ± 6 |
| (55–73) | (54–76) | (53–71) | (55–73) | (52–74) | |
| LV mass (g) | 90 ± 13 † , ‡ , § , ∥ | 107 ± 17 ⁎ , § , ∥ | 117 ± 20 § | 145 ± 23 ⁎ , † , ‡ | 130 ± 16 ⁎ , † |
| (53–121) | (82–159) | (78–147) | (105–196) | (95–173) | |
| LV mass index (g/m 2 ) | 55 ± 7 † , ‡ , § , ∥ | 64 ± 7 ⁎ , § , ∥ | 77 ± 9 ⁎ , § , ∥ | 76 ± 8 ⁎ , † , ‡ | 76 ± 4 ⁎ , † . ‡ |
| (34–77) | (52–84) | (54–91) | (60–98) | (60–92) | |
| LVRI (g/ml) | 1.00 ± 0.05 | 0.99 ± 0.04 | 1.04 ± 0.04 | 0.99 ± 0.08 | 0.99 ± 0.06 |
| (0.85–1.07) | (0.90 ± 1.06) | (0.97–1.17) | (0.87–1.19) | (0.79–1.09) | |
| SDI (%) | 1.36 ± 0.40 | 1.21 ± 0.28 | 1.10 ± 0.51 | 1.02 ± 0.51 | 1.07 ± 0.43 |
| (0.46–2.38) | (0.39–1.86) | (0.74–1.60) | (0.42–2.08) | (0.46–2.63) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


