Patients with truncus arteriosus often require pulmonary arterial (PA) and/or right ventricular outflow tract (RVOT) reintervention within the first year of repair. However, little is known about the risk factors for early reintervention on the PAs or RVOT in this population. The objective of the present retrospective cohort study was to determine the risk factors for early PA or RVOT reintervention after repair of truncus arteriosus in neonates and young infants. Of 156 patients ascertained (median age at repair 14 days; 143 early survivors), reinterventions on the RVOT and/or PAs were performed in 109. The first reintervention was catheter therapy in 73 patients (conduit dilation/stenting in 29, PA dilation/stenting in 31, both in 13) and conduit reoperation in 36 patients. The freedom from any RVOT or PA reintervention was 68 ± 4% at 1 year and 48 ± 5% at 2 years. The factors associated with early reintervention (shorter 1-year freedom from reintervention) on univariate analysis were repair quartile, neonatal repair, smaller weight at repair, and smaller implanted conduit size. On multivariable analysis, only smaller conduit size remained significant (multivariable hazard ratio 0.66/mm, range 0.53 to 0.83; p <0.001). The freedom from conduit reoperation was 92 ± 3% at 1 year and 76 ± 4% at 2 years. Overall, the left and right PA sizes were modestly larger than normal by the 1-sample t test, and PA Z scores and the PA area index were not associated with the risk of reintervention. Early reintervention for PA and/or RVOT conduit obstruction is common after neonatal and early infant repair of truncus arteriosus using homograft conduits. A smaller conduit size was associated with early RVOT/PA reintervention. The branch PA size was normal before surgery, suggesting that the PA stenosis in these patients resulted from factors other than intrinsic stenosis or hypoplasia.
In truncus arteriosus, the PAs originate directly from the ascending aorta. The repair of truncus arteriosus involves reconstruction of the right ventricular outflow tract (RVOT), along with repair of the ascending aortic defect from which the PAs were removed and closure of the ventricular septal defect. In our experience, reinterventions on the RVOT and/or PAs are frequently necessary in infancy or early childhood in patients with truncus arteriosus, including balloon or surgical pulmonary arterioplasty and conduit dilation, stenting, or surgical revision/replacement. In patients who undergo RVOT conduit placement for repair of conotruncal anomalies, including truncus arteriosus, factors associated with shorter freedom from conduit reintervention have been investigated in a number of studies. At least 1 group has found that, among young patients repaired with RVOT conduits, truncus arteriosus is a risk factor for earlier reintervention. However, limited data are available specifically concerning the risk factors for early reintervention on the branch PAS or RVOT after truncus repair. It has been our impression that very early pulmonary arterial (PA) or RVOT reintervention (<1 year postoperatively) is particularly common after truncus repair. A deeper understanding of the anatomic or procedural factors associated with this outcome might help modify practice to improve the freedom from PA or RVOT reintervention.
Methods
Patients who underwent repair of truncus arteriosus at Children’s Hospital Boston during the first 6 months of age, from January 1989 through December 2008, were ascertained from the computer database of the Department of Cardiology. Patients with associated congenital cardiovascular anomalies were included, unless the associated anomalies resulted in a functionally univentricular circulation (e.g., tricuspid atresia). The Children’s Hospital committee on clinical investigation approved the protocol.
Cross-sectional follow-up was obtained by April 2010 in an effort to ensure that patients had a minimum potential follow-up of 1 year. The indications for RVOT/PA reintervention were not standardized and might have varied according to era, patient age, clinical status, referring physician, and catheterizing physician. The study began with patients who underwent surgery in 1989, because transcatheter PA angioplasty was well established at our center, and stents were first introduced.
Instead of classification by “truncus type,” the PA anatomy was categorized using a modified system as (1) confluent branch PAs with a main PA segment, (2) confluent branch PAs without a main PA segment, or (3) nonconfluent branch PAs. The branch PAs were measured on preoperative echocardiography by a single reviewer who was unaware of the patient outcome. Z scores for the proximal PA branches were calculated from previously published normative data from our center. A modified PA area index was derived by calculating the cross-sectional area of the left and right PAs from the echocardiographic diameters (circular cross section assumed), summing the areas, and indexing them to the body surface area.
The primary outcome measure was freedom from early (<1 year) surgical or transcatheter reintervention on the RVOT or PAs. For the present analysis, Kaplan-Meier and Cox regression analysis were performed, because a full year of follow-up was not available for all patients. Patients alive without reintervention at 1 year after repair were censored event-free at that time. The patients who had died before 1 year were censored event-free at the time of death. The secondary outcomes included freedom from any surgical or transcatheter RVOT or PA reintervention (not limited to 1 year; patients were censored event-free at death or last follow-up if they had not undergone reintervention) and freedom from transcatheter PA dilation or stenting (patients censored event-free at surgical reintervention, death, or last follow-up). For freedom from event analysis, hazard ratios (HRs) are presented with the 95% confidence intervals. The predictor variables included age at repair, year/era of repair (procedure quartile), PA anatomy, the PA area index, the average of native (unrepaired, before repair) left and right proximal PA Z scores, individual PA diameters and Z scores, conduit type and size, conduit Z score (based on normative pulmonary valve annulus data at repair), and presence/repair of interrupted aortic arch. The branch PA Z scores were compared to normal ( Z score of 0) using a 1-sample t test.
Results
From 1989 to 2008, 156 patients underwent truncus arteriosus repair during the first 6 months of age at Children’s Hospital Boston. There were 13 early deaths (within 1 month of surgery; 8%), 10 during the first half of the study period. The patient-related and surgical data are listed in Table 1 . Homograft conduits were used in all but 1 patient in the present series. From the first quartile to the last quartile of this experience, the age at repair decreased significantly, from a median of 43 days to 10 days (p <0.001).
| Variable | Value |
|---|---|
| Age (days) | |
| Median | 14 |
| Range | 2–170 |
| Age group (n) | |
| ≤30 d | 109 (70%) |
| 31–90 d | 29 (19%) |
| 91–180 d | 18 (12%) |
| Weight (kg) | |
| Median | 3.2 |
| Range | 1.1–9.7 |
| Associated cardiovascular anomalies | |
| None | 93 (60%) |
| Coarctation/interrupted aortic arch | 28 (18%) |
| Coronary anomalies | 42 (27%) |
| Pulmonary arterial anatomy | |
| Confluent, main pulmonary arterial segment present | 89 (57%) |
| Confluent, no main pulmonary arterial segment | 59 (38%) |
| Nonconfluent | 8 (5%) |
| Conduit type | |
| Aortic homograft | 87 (56%) |
| Pulmonary homograft | 69 (44%) |
| Conduit size (mm) | |
| Median | 10 |
| Range | 6–17 |
| Conduit size group (n) | |
| <10 mm | 65 (42%) |
| 10–12 mm | 74 (47%) |
| >12 mm | 17 (11%) |
| Z score ⁎ | |
| Median | 1.3 |
| Range | −1.6 to 6.1 |
⁎ Conduit Z score calculated from nominal conduit diameter, according to normal pulmonary valve annulus measurements. For example, 10-mm conduit in a patient with a body surface area of 0.20 m 2 would have a Z score of 1.54.
The branch PAs were confluent in 148 patients (95%), 89 of whom had a main PA segment. Of the 8 patients with nonconfluent branch PAs, all had an origin of 1 PA branch from the common trunk, with the other being the other lung supplied by either a single PA branch arising from a ductus arteriosus or ductal remnant (n = 5) or by multiple systemic-to-PA collaterals (n = 3).
The preoperative PA diameters, Z scores, and PA area indexes are listed in Table 2 . Overall, the left and right PA sizes were modestly larger than normal using the 1-sample t test. Only 8 patients had Z scores <−2 (less than the normal range) for 1 (n = 6) or both (n = 2) branch PAs. The PA Z scores were not associated with age at repair, any of the other demographic or anatomic variables assessed, or the diameter of the implanted conduit.
| Measurement | Mean ± SD | p Value ⁎ |
|---|---|---|
| Left pulmonary artery | ||
| Diameter (cm) | 0.54 ± 0.16 | — |
| Z score | 0.64 ± 1.74 | <0.001 |
| Right pulmonary artery | ||
| Diameter (cm) | 0.56 ± 0.16 | — |
| Z score | 0.50 ± 1.69 | <0.001 |
| Pulmonary artery area index (mm 2 /m 2 ) | 296 ± 165 | — |
⁎ One-sample t test, comparison with normal mean Z score = 0.
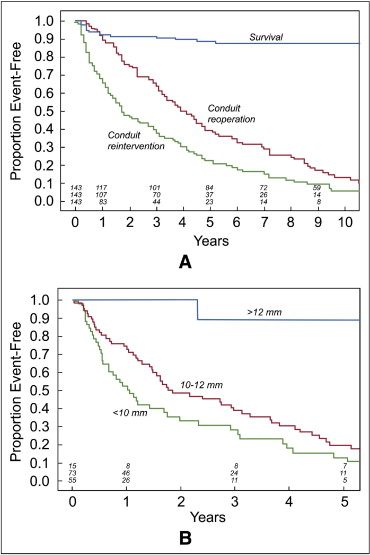
During a median follow-up of 7.1 years (range 1 month to 21 years), 16 additional patients died, 10 within the first postoperative year. The postdischarge survival among the 143 patients who lived for ≥1 month after repair and to hospital discharge was 92 ± 2% at 1 year and 89 ± 3% at 5 years ( Figure 1 ); including the early deaths, the survival was 85 ± 3% at 1 year and 81 ± 3% at 5 years.
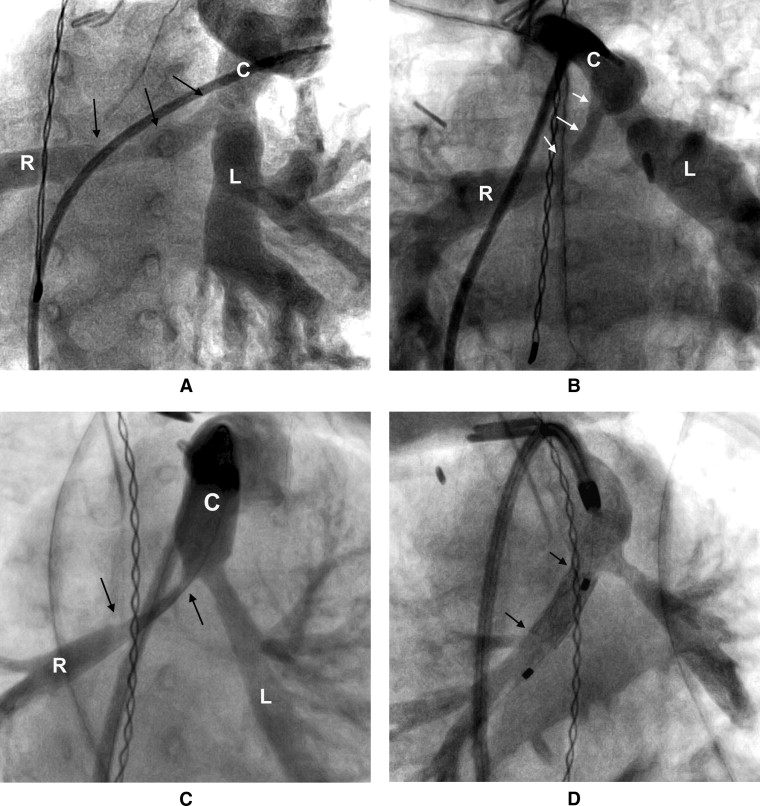
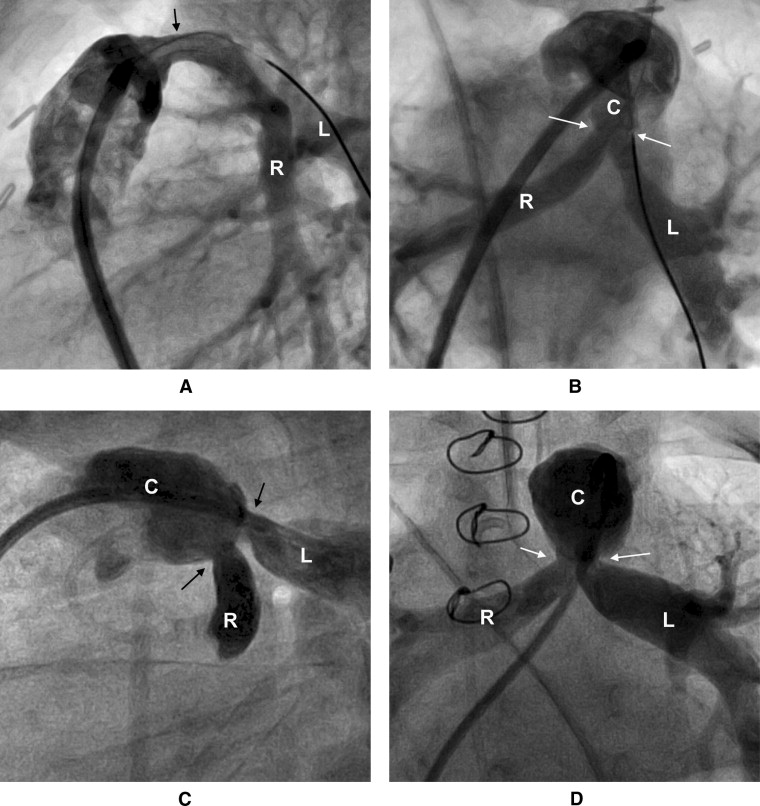
Reinterventions on the RVOT and/or PAs were performed in 109 patients. The types and details of the first reintervention are summarized in Table 3 and depicted in a competing risk curve ( Figure 1 ). As demonstrated in Figure 2 , the stenosis of the right PA often appeared to be over a long segment, running from the origin along its retroaortic course. In patients with both PA and conduit stenosis, the stenosis tended to involve the conduit-to-PA anastomosis and 1 or both proximal branch PAs ( Figure 3 ). The freedom from transcatheter PA dilation or stenting without previous or concomitant conduit reintervention was 84 ± 3% at 1 year and 76 ± 4% at 2 years. No patient-related or surgical factors were associated with a shorter freedom from this outcome.
| Variable | Patients (n) |
|---|---|
| Method of first right ventricular outflow tract or pulmonary arterial reintervention | |
| Any reintervention | 109 |
| First reintervention, surgical | 36 |
| Conduit replacement | 25 |
| Conduit augmentation | 11 |
| With branch pulmonary arterioplasty | 18 |
| First reintervention, catheter based | 73 |
| Conduit dilation or stenting only | 29 |
| Pulmonary arterial dilation or stenting only | 31 |
| Conduit and pulmonary arterial dilation or stenting | 13 |
| Details of first catheter-based reintervention | |
| First reintervention included conduit dilation or stenting | 42 |
| Conduit stenting | 25 |
| Conduit dilation without stenting | 17 |
| First reintervention included pulmonary arterial dilation or stenting | 44 |
| Unilateral dilation or stenting | 24 |
| Bilateral dilation or stenting | 20 |
| Right pulmonary arterial dilation or stenting | 39 |
| Right pulmonary arterial stenting | 12 |
| Right pulmonary arterial dilation without stenting | 27 |
| Left pulmonary arterial dilation or stenting | 25 |
| Left pulmonary arterial stenting | 2 |
| Left pulmonary arterial dilation without stenting | 23 |
The freedom from any surgical or transcatheter RVOT or PA reintervention was 68 ± 4% at 1 year, 48 ± 5% at 2 years, and 23 ± 4% at 5 years ( Figure 4 ). A smaller conduit size at implantation was the only factor associated with shorter freedom from conduit or PA reintervention on univariable and multivariable analysis, both unadjusted (HR 0.77 per millimeter of conduit diameter, range 0.69 to 0.81; p <0.001) and adjusted for era quartile and age (HR 0.71, range 0.62 to 0.82; p <0.001; Figure 4 ). The results of univariable analysis for our primary outcome, reintervention within 1 year of repair, are summarized in Table 4 . For the present analysis, the patients who were alive without reintervention 1 year postoperatively were censored event-free at that point. On univariable analysis, the era of repair, neonatal repair, lower weight at repair, conduit diameter, and conduit diameter Z score were associated with 1-year freedom from reintervention on univariable. On multivariable analysis, including these independent variables, only the conduit diameter remained significantly associated with 1-year freedom from RVOT or PA reintervention (HR 0.66 per millimeter, range 0.53 to 0.83; p <0.001). Owing to the co-linearity of the conduit diameter and Z score, the final multivariate analysis was performed without inclusion of the conduit size Z score.