Chapter 2 Advances in 3D echocardiography technology have ushered its use into mainstream clinical practice. 1 3D echocardiography offers a realistic, multiplanar image of both valves and their spatial relationships with adjacent structures, providing anatomic and functional insight that has furthered our understanding of normal spatial relationships and the anatomic and functional abnormalities that develop in patients with valvular heart disease. The mitral annulus is a fibromuscular ring to which the anterior and posterior mitral valve leaflets attach. The normal mitral valve annulus has a 3D saddle shape with its “lowest points” at the level of both commissures. This allows proper leaflet apposition during systole and minimizes leaflet stress. 2 The annulus can be divided into the anterior and posterior annulus based on the insertion of the corresponding leaflets. The anterior portion of the annulus attaches to the right and left fibrous trigones. The right trigone is a fibrous area situated between the membranous septum, the mitral valve, the tricuspid valve, and the noncoronary cusp at the aortic annulus. The left trigone is a fibrous area located at the nadir of the left coronary cusp of the aortic annulus and the left border of the aortic-mitral curtain. The aortic-mitral curtain is the fibrous tissue between the anterior mitral valve leaflet, the left and non-coronary cusps of the aortic valve, and the left and right trigone. 3 The posterior portion of the annulus is less developed owing to the discontinuity of the fibrous skeleton of the heart in this region. This difference explains why the posterior portion of the mitral annulus is more prone to pathologic dilation and the anterior portion is relatively resistant. 4 The annulus is a dynamic structure that undergoes 3D deformation in its circumference, excursion, curvature, shape, and size for proper function, which makes it susceptible to ventricular remodeling.5–9 The mitral valve has an anterior and a posterior leaflet ( Figure 2-1). The atrial, or smooth, surface is free of any attachments whereas the ventricular, or rough, surface connects to the papillary muscles via the chordae tendinae. The posterior leaflet, which is quadrangular, is attached to approximately three fifths of the annular circumference. The anterior leaflet has a semicircular shape and is attached to approximately two fifths of the annular circumference. 10 Although the posterior leaflet attaches to a larger portion of the annular circumference, it is shorter than the anterior leaflet. FIGURE 2-1 Anatomic relationship of the aortic and mitral valves. There are two major terminology classifications for the segmental anatomy of the mitral leaflets, which help with the description of the localization of specific mitral valve lesions. The leaflet segmentation scheme proposed by Carpentier 11 is the most widely used. In this scheme, the posterior leaflet has two well-defined indentations dividing it into three separate sections or “scallops.” The anterolateral scallop is defined as P1, the middle scallop as P2, and the posteromedial scallop as P3. The anterior leaflet typically has a smoother surface and is devoid of indentations. The segment of the anterior leaflet opposing P1 is designated A1 (anterior segment), the segment opposite to P2 is A2 (middle segment), and the segment opposite to P3 is A3 (posterior segment) ( Figure 2-2). FIGURE 2-2 Classification of mitral valve segmental anatomy. The modified Duran nomenclature is based on the chordal insertion of the papillary muscles. 12 In this classification, the posterior leaflet is divided into P1, PM1, PM2, and P2 and the anterior leaflet is divided into A1 and A2. If a line were drawn directly down the center of the mitral valve, then P1, PM1, and A1 would be grouped together because they all attach to the anterolateral papillary muscle, and P2, PM2, and A2 would be grouped together because they attach to the posteromedial papillary muscle (see Figure 2-2). A final proposed classification is the modified Carpentier classification, which is a combination of both the Carpentier and the modified Duran nomenclature. 13 This classification scheme divides A2 and P2 into medial (M) and lateral (L) segments, grouping P1, P2L, A1, and A2L because they are attached to the anterolateral papillary muscle and P2M, P3, A2M, and A3 because they are attached to the posteromedial papillary muscle. This last terminology converts the well-known Carpentier leaflet segmentation into anatomically relevant groupings (see Figure 2-2). During systole, the margins of the two mitral leaflets oppose each other for several millimeters to ensure valve competency against normal left ventricular (LV) end-systolic pressure.14,15 The distinct area where the anterior and posterior leaflets appose each other during systole is known as the commissure. Carpentier divides the commissures into anterolateral and posteromedial commissures. 11 The amount of tissue in the commissures varies from several millimeters of leaflet tissue to distinct leaflet segments. The chordae tendinae are responsible for determining the position and tension on the anterior and posterior leaflets at LV end-systole. The chordae are fibrous extensions originating from the heads of the papillary muscles and infrequently from the inferolateral ventricular wall. They are named according to their insertion site on the mitral leaflets. Marginal or primary chordae insert on the free margins of the mitral leaflets and prevent marginal prolapse. Intermediate or secondary “strut” chordae insert on the ventricular surfaces of the leaflets, preventing billowing while reducing tension on the leaflet tissues.16,17 These chords may also play a role in determining dynamic ventricular shape and function through their contribution to ventricle-valve continuity.18,19 Basal or tertiary chordae insert on the posterior leaflet base and mitral annulus. Their specific function is unclear. The two papillary muscles—the anterolateral and the posteromedial—originate from the area between the apical and middle thirds of the LV free wall. The anterolateral papillary muscle has an anterior head and a posterior head, whereas the posteromedial papillary muscle usually has anterior, intermediate, and posterior heads. 20 The anterolateral papillary muscle has a dual blood supply from both the left anterior descending and left circumflex arteries, and the posteromedial papillary muscle receives its single blood supply from the right coronary artery when the right coronary is dominant, which is the situation in 90% of individuals. When the left coronary is dominant, the posteromedial papillary muscle is supplied by the left circumflex. Because the papillary muscles connect directly to the left ventricle, any geometric alteration of the ventricle can change the axial relationship of the chordae and leaflets, resulting in poor leaflet coaptation. In addition to its ability to provide detailed and multidimensional images of the mitral valve, 3D echocardiography also provides accurate and reproducible quantification of mitral valve geometry and dynamics throughout the cardiac cycle. With the advent of 3D imaging, new parameters of annular, coaptation, leaflet, and subvalvular geometry are easily obtained.3,10 These measurements have generated new insights into the mechanics of the mitral valve. A detailed description of the most commonly used parameters is shown in Figures 2-3 and 2-4. FIGURE 2-3 Volumetric reconstruction of the mitral valve. FIGURE 2-4 Functional anatomy of the mitral annulus during the cardiac cycle. Diseases that affect the mitral valve are best described by defining the etiology of the disease, the specific lesions caused by the disease, and the dysfunction it creates on the mitral valve apparatus. This “pathophysiologic triad,” first described by Carpentier et al 21 in the early 1980s, is still extremely useful today in characterizing different types of mitral valve disorders. These lesions, in turn, lead to mitral valve dysfunction. Instead of classifying this dysfunction as simply mitral stenosis (MS) or MR, Carpentier 11 developed a classification scheme to aid in the surgical strategy on the basis of the type of leaflet motion ( Table 2-1). Patients with mitral annular dilation or leaflet perforation usually have normal leaflet motion and are categorized as having type I dysfunction. Type II dysfunction consists of prolapse (free edge of one or both leaflets overriding the plane of the annulus during valve closure) and flail (excessive motion of the leaflet margin above the plane of the annulus) due to excessive and redundant leaflet tissue and chordal rupture, respectively ( Figure 2-5). Leaflet restriction during valve closure due to fusion of various components of the mitral valve apparatus is defined as type IIIa dysfunction, whereas leaflet restriction during valve opening resulting from leaflet tethering, is defined as type IIIb dysfunction. FIGURE 2-5 Myxomatous disease with mitral valve prolapse. Mitral valve prolapse is now recognized as the most common cause of MR in developed countries. 22 It results primarily from two distinctive types of degenerative diseases, Barlow disease and fibroelastic deficiency ( Table 2-2). There is considerable overlap between these two entities, and it is difficult to reliably distinguish them on the basis of either the gross or histologic appearance of the valve. Some valves may represent a forme fruste of Barlow disease and demonstrate myxoid infiltration on subsequent histologic examination. 6 TABLE 2-2 Key Differences Between Barlow Disease and Fibroelastic Deficiency Barlow disease results from an excess of myxomatous tissue, which is an abnormal accumulation of mucopolysaccharides in one or both of the leaflets and many or few of the chordae. 23 This myxoid infiltration results in thick, bulky, redundant, billowing leaflets and elongated chordae, which often lead to bileaflet, multisegmental prolapse ( Figure 2-6). Barlow disease is usually diagnosed in young adulthood, and patients are typically monitored for many decades with well-preserved LV size until criteria for surgery are met in the fourth or fifth decade of life. FIGURE 2-6 Barlow disease and fibroelastic deficiency. In contrast, fibroelastic deficiency results from acute loss of mechanical integrity due to abnormalities of connective tissue structure and/or function. 23 It usually leads to either a localized or unisegmental prolapse due to elongated chordae or flail leaflet due to ruptured chordae (see Figure 2-6). Patients most commonly present in the sixth decade of life with a relatively short history of MR. This entity is the most common form of organic mitral valve disease for which mitral valve repair surgery is required. There is considerable overlap between these two entities and it is difficult to reliably distinguish them based on either the gross or histologic appearance of the valve. Some valves may represent a forme fruste of Barlow disease and will demonstrate myxoid infiltration on subsequent histological examination. 6 Ischemic MR is a pathophysiologic outcome of ventricular remodeling arising from ischemic heart disease. The adverse changes that occur in the ventricle after an ischemic event commonly result in type IIIB dysfunction of the mitral valve with leaflet restriction during systole. 11 Ischemic MR occurs in approximately 20% to 25% of patients with myocardial infarction even in the era of reperfusion, and these patients have significantly worse outcomes irrespective of the severity of MR. 24 The resultant volume overload caused by MR worsens myocardial contractility, which in turn worsens LV dysfunction, eventually leading to heart failure and death.25–28 As mentioned earlier, the mitral valve is dynamic and changes from a saddle shape (hyperbolic paraboloid) during systole to a flatter configuration during diastole. During systole, competing forces act on the mitral valve leaflets. Increased LV pressure acts to push the leaflets toward the left atrium while tethering forces from the chordae act to pull the leaflets in the direction of the left ventricle. The saddle shape morphology is believed to balance these forces by optimizing leaflet curvature, and, thus, minimizing mitral leaflet stress. 2 In the setting of a myocardial infarction and resultant LV remodeling, an outward and apical displacement of the posteromedial papillary muscle occurs, which tethers the mitral valve leaflets into the left ventricle, restricting their ability to coapt effectively at the level of the mitral annulus. 29 Furthermore, the mitral annulus dilates, making leaflet coaptation even more difficult ( Figures 2-7 and 2-8). 30 FIGURE 2-7 Papillary muscle orientation in secondary forms of mitral regurgitation.
Three-Dimensional Anatomy of the Aortic and Mitral Valves
Mitral Valve Anatomy
Mitral Annulus
Mitral Valve Leaflets
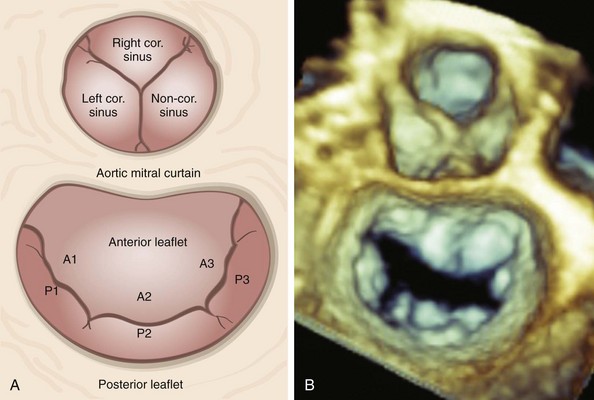
A, Schematic diagram and B, en face three-dimensional echocardiographic zoom-mode image of the mitral valve from the left atrial, or the surgeon’s, perspective depicting typical anatomic relationships. In this view, the aortic valve occupies the 12 o’clock position. The aortic mitral curtain separates the anterior leaflet from the aortic valve. A1 to A3, Anterior leaflet segments; cor., coronary; P1 to P3, posterior leaflet segments.
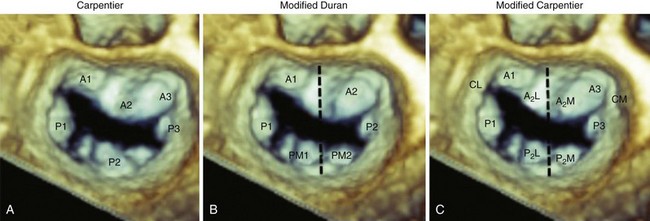
A, The Carpentier system divides the posterior leaflet into three scallops (P1, P2, P3) on the basis of leaflet indentation. The anterior leaflet is then divided and classified into three segments on the basis of their relationship to the posterior leaflet (A1, A2, A3). B, The Duran system divides the leaflet on the basis of the chordal insertion of the papillary muscles. Thus the anterior leaflet is divided into two segments (A1, A2) and the posterior into four segments (P1, PM1, P2, PM2). Segments A1, P1 and PM1 attach to the anterolateral papillary muscle, and segments A2, P2, and PM2 to the posteromedial papillary muscle. C, The modified Carpentier system divides A2 and P2 into lateral (A2L, P2L) and medial (A2M, P2M) segments, allowing grouping of leaflet segments according to papillary muscle attachment. Thus, segments A1, A2L, P1, and P2L are attached to the anterolateral papillary muscle, and A3, A2M, P3, and P2M to the posteromedial papillary muscle.
Mitral Valve Commissures
Mitral Valve Chordae
Papillary Muscles
Mitral Valve Apparatus Quantification
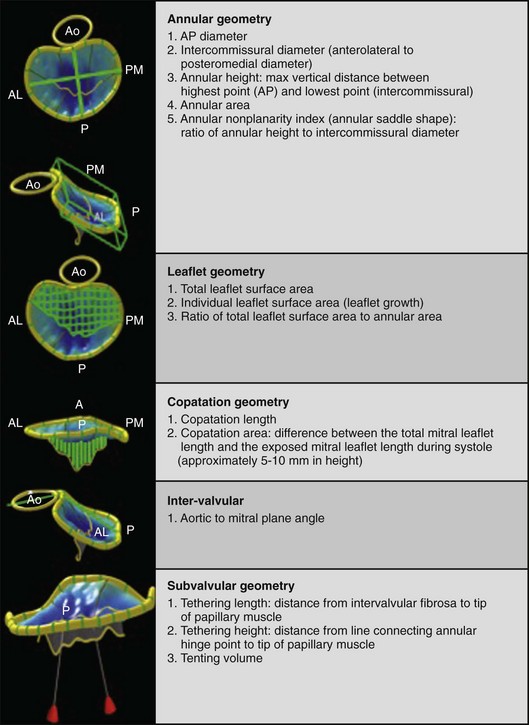
3D echocardiography–based software provides measurements of mitral annular, leaflet, coaptation line, intervalvular relationships, and subvalvular geometry. A, Anterior; AL, anterolateral; Ao, aortic valve; P, posterior; PM, posteromedial.
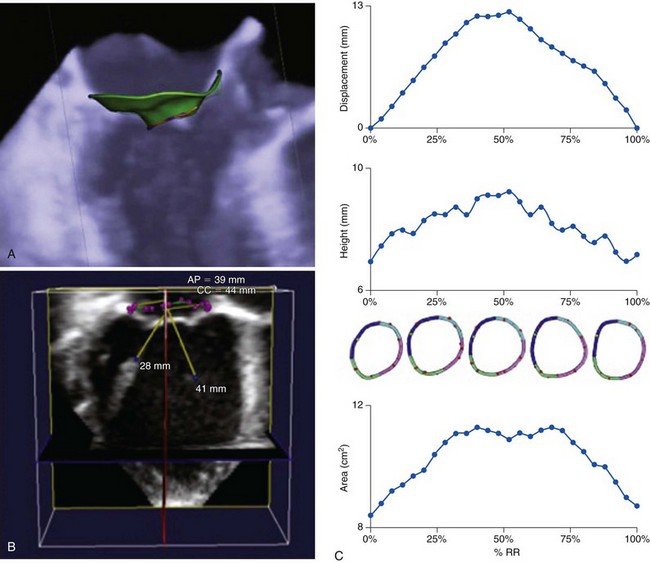
A, Three-dimensional transesophageal echocardiography image of the mitral valve with a three-dimensional mitral valve model of the annulus, leaflets, and coaptation line imposed on the image. B, three-dimensional transesophageal echocardiographic dataset of the mitral valve with a three-dimensional mitral valve model of the mitral annulus and papillary muscles superimposed on the image. C, Results from specialized three-dimensional echocardiographic software tracking mitral annular displacement, height, and area during systole. AP, Anteroposterior; CC, inter-commisural; RR, R-R interval.
Mechanisms of Mitral Valve Dysfunction
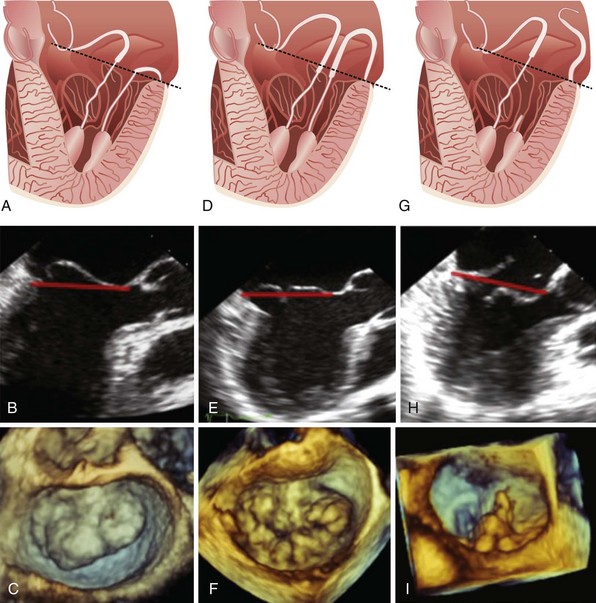
A, Schematic demonstrating anterior leaflet prolapse. B, Imaging from two-dimensional transesophageal echocardiography. C, three-dimensional transesophageal echocardiographic image of the mitral valve with anterior leaflet prolapse as viewed en face from the left atrium. Leaflet prolapse is diagnosed when the free edge of the leaflet overrides the plane of the mitral annulus during systole. D, Schematic demonstrating bileaflet billowing of the mitral valve due to chordae elongation. E, Images of bileaflet mitral valve billowing from two-dimensional transesophageal echocardiography and F, three-dimensional transesophageal echocardiography as viewed en face from the left atrium. Leaflet billowing is diagnosed when there is systolic excursion of the leaflet body into the left atrium as a result of excess leaflet tissue, with the leaflet free edge remaining below the plane of the mitral annulus. G, Schematic demonstrating anterior mitral leaflet prolapse and posterior mitral leaflet flail due to chordal rupture. H, A two-dimensional transesophageal echocardiographic example of P2 flail segment. I, Corresponding three-dimensional transesophageal echocardiographic en face image as viewed from the left atrium. The dashed black and solid red lines in parts A to H represents the mitral annular plane.
Degenerative Mitral Valve Disease
DIFFERENTIATING CHARACTERISTICS
BARLOW DISEASE
FIBROELASTIC DEFICIENCY
Pathology
Excess of myxomatous tissue
Impaired production of connective tissue
Typical age at diagnosis
Younger (<40 years)
Older (>60 years)
Duration of disease
Years to decades
Days to months
Physical findings
Midsystolic click and late systolic murmur
Holosystolic murmur
Leaflet involvement
Multisegmental
Unisegmental
Leaflet lesions
Leaflet billowing and thickening
Thin leaflets with a thickened involved segment
Chordae lesions
Chordal thickening and elongation
Chordal elongation and chordal rupture
Carpentier classification
Type II
Type II
Type of dysfunction
Bileaflet prolapse
Prolapse and/or flail
Complexity of valve repair
More complex
Less complex
Barlow Disease
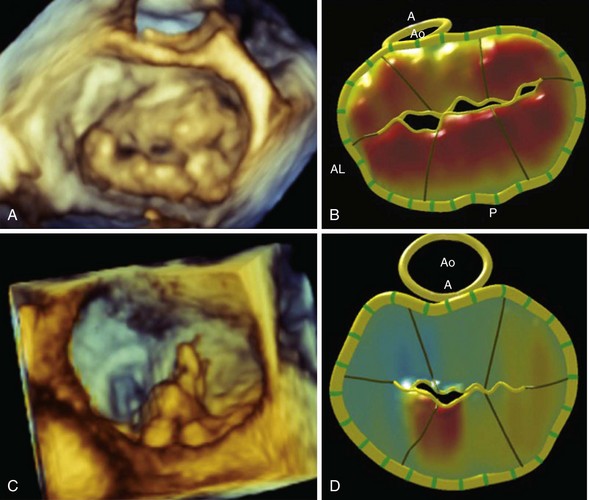
A, En face three-dimensional transesophageal echocardiographic image of a mitral valve as viewed from the left atrium, demonstrating bileaflet prolapse in a patient with Barlow disease. B, The corresponding parametric map, in which the color gradations towards orange indicate the distance of the leaflet from the mitral annular plane toward the left atrium. C, En face three-dimensional transesophageal echocardiographic image of a mitral valve as viewed from the left atrium, demonstrating a flail P2 segment due to fibroelastic deficiency. D, The corresponding parametric map demonstrates the abnormal P2 segment. A, anterior; AL, anterolateral; Ao, aortic valve; P, posterior.
Fibroelastic Deficiency
Ischemic Mitral Regurgitation
Mechanism of Ischemic Mitral Regurgitation
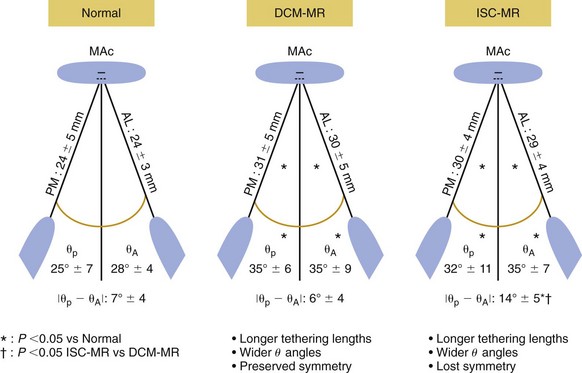
The papillary muscles in patients with mitral regurgitation (MR) secondary to dilated cardiomyopathy (DCM) or ischemia (ISC) are longer than those in patients with normal mitral valves. The increase in papillary muscle length is symmetric in patients with dilated cardiomyopathy, but in patients with ischemic mitral regurgitation, the increase in papillary muscle length is asymmetric with a greater increase in the posteromedial (PM) than the anteriolateral (AL) papillary muscle. θP and θA are the angles between the left ventricular long-axis and the line connecting the mitral annular center (MAC) to the tip of the posteromedial and anterolateral papillary muscles respectively. *P <0.05![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Three-Dimensional Anatomy of the Aortic and Mitral Valves
Only gold members can continue reading. Log In or Register to continue