Thoracoplasty: Indications and Surgical Considerations
Stanley C. Fell
The term thoracoplasty (TPL) refers to a spectrum of operations designed to reduce the volume of a hemithorax. This is achieved by multiple rib resections, allowing collapse of the chest wall and apposition of the parietal to the visceral or mediastinal pleura. These procedures have been used to obliterate a septic pleural space overlying unexpandable lung, to treat postpneumonectomy empyema, to compress cavitary pulmonary tuberculosis, and to reduce the pleural space when there is inadequate expansion of residual lung following pulmonary resection.
Historical Context
A detailed description of the development of TPL and its permutations is beyond the scope of this chapter; it may be found in the writings of Alexander,2 Hochberg,12 and Meade,24 from which this brief narrative is gleaned. Wide decostalization of the chest wall was employed by Estlander and Schede27 for the treatment of chronic empyema in the late nineteenth century, and the latter’s procedure was used until the mid-twentieth century, when it was virtually abandoned because it resulted in severe physiologic and cosmetic deformities. It was replaced, when feasible, by decortication.
In 1819, Carson suggested that induced pneumothorax might promote healing of the diseased lung. Forlanini, in 1882, having observed that clinical improvement sometimes followed when spontaneous pneumothorax occurred in pulmonary tuberculosis, proposed and used artificial pneumothorax to collapse and compress these cavities. When induction of pneumothorax was unsuccessful because of adhesions, intrapleural pneumolysis was developed by Jacobeus in 1913. Other methods used to collapse the lung—such as phrenic nerve exeresis, pneumoperitoneum, and paraffin plombage—were inconsistently successful. In 1885, de Cerenville collapsed tuberculous cavities by resecting portions of the second and third ribs. In 1907, Friedrich resected ribs 2 through 9 in one stage. This procedure had an unacceptable level of morbidity and mortality even in its modifications by Sauerbruch. It remained for John Alexander, bedridden with spinal tuberculosis (Pott’s disease) in 1925, to analyze the shortcomings of prior procedures and develop the operation, as revised by Langston,21 that is used to this day.
Indications for Thoracoplasty
In cases of tuberculosis, TPL was an operation suited for cavities in the upper portion of the lung, typically the apical and posterior segments of the upper lobe and the superior segment of the lower lobe. Large cavities in the apex of the lung and those situated medially in the paravertebral gutter are difficult to collapse by TPL. Cavities ≥5 cm in diameter often could not be closed by TPL, and those that were distended because of partial bronchial obstruction (tension cavities) were also not responsive. Tuberculous bronchiectasis is another contraindication to TPL. Bilateral TPL is rarely performed but may be feasible if limited to three to five ribs on each side. Alexander2 reported cavitary closure in 93% of survivors, with a 10% mortality rate.
Indications for TPL have diminished considerably since the advent of decortication for unexpanded lung, intrathoracic muscle flap transposition for the treatment of empyema and bronchopleural fistula, and antibiotic therapy and pulmonary resection for tuberculosis. However, these procedures may fail. For example, decortication is doomed to failure if the lung is fibrotic and inexpansible. TPL can provide a simple one-stage solution to a vexing problem. In cases where pulmonary resection for advanced lung cancer has been performed and complicated by bronchopleural fistula or empyema, the choice of muscle flap procedures versus TPL must be considered. If the long-term prognosis for the patient is poor, he or she may be better served by a one-stage TPL rather than a multiple-stage muscle flap procedure and a prolonged period of hospitali- zation.24,25
To be effective, the classic extrapleural TPL requires that the parietal pleura be thin and pliable in order to obliterate the pleural space. This is not a relevant problem in the management of early bronchopleural fistula (BPF) and empyema. In chronic empyema, however well drained, the parietal pleura becomes so thick and stiff that space obliteration will not occur. Thus, the Grow,11 Kergin,20 and Andrews3 modifications of the TPL evolved.
Alexander Thoracoplasty for Tuberculosis
In the last analysis, a thoracoplasty is used to close a pulmonary cavity that no other operations can close.
—John Alexander, 1937
The posterolateral TPL was originally performed in three stages, separated by 2 to 3 weeks in order to minimize the adverse effects of paradoxical chest wall motion on ventilation and on the ability to cough and expectorate (Fig. 65-1). With the advent of antibiotic therapy for tuberculosis in 1945, and thus more localized disease, the stages were reduced to two. When TPL is performed for postpneumonectomy empyema, BPF, or incomplete lung expansion following lobectomy, it is a one-stage operation. The absence of lung under the TPL prevents the effects of chest wall paradoxic motion.
Method
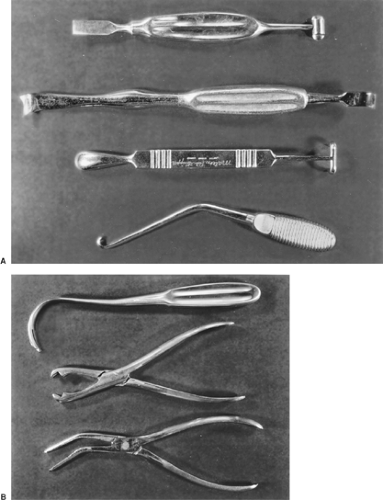
With the patient in the true lateral position under endotracheal anesthesia, heed Alexander’s instruction: “The uppermost arm should hang over the edge of the table so as to assist by its weight in the forward displacement of the scapula.” The arm is protected by padding. Under no circumstances allow the anesthesiologist to fix or suspend the upper limb, since by so doing the scapula will invariably overhang and obscure the operative field. What follows is the technique of the Alexander TPL that I used in the 1950s in cases of sputum-positive pulmonary tuberculosis in patients deemed at high risk for resection by reason of extent of disease or associated illness. Because several generations of thoracic surgeons have been trained in intercostal and muscle-sparing thoracotomies and have had little or no experience in the instrumentation required for TPL, the following may be of value. The Coryllos periosteal elevator is ideal for stripping the rib and invaluable for freeing the necks of ribs and the transverse processes. The Matson or Cameron Haight elevators are used for stripping both surfaces of the first rib (Fig. 65-2A). A sequestrum or lion-jaw bone-holding forceps is required to grasp the first rib after it is transected posteriorly (Fig. 65-2B). The ribs are transected with the conventional Bethune rib shears, and the Sauerbruch box-nosed rongeur is used to resect the rib necks and the transverse processes.
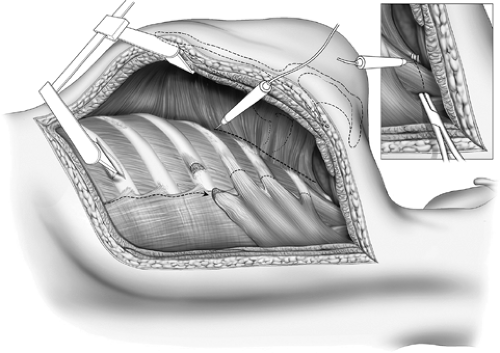
A periscapular incision is made commencing at the level of the first dorsal vertebra midway between the scapula and the spine and carried to two finger breadths below the angle of the
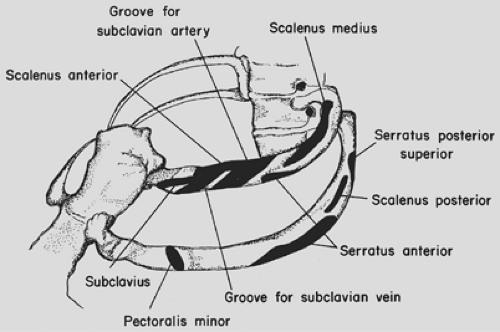
scapula (Fig. 65-3). The trapezius, rhomboids, and upper portion of the latissimus dorsi muscles are divided in the line of the incision. If the use of the latissimus as an intrathoracic muscle flap is contemplated, it may be mobilized instead of being incised. The scapula is elevated. A 3-cm incision is made in the periosteum on the upper border of the sixth rib, and this periosteum is elevated, thus allowing a flange of a rib spreader to be inserted (Fig. 65-4). The rib spreader is opened and the upper flange elevates and fixes the scapula, thus freeing the assistant for other duties. The attachments of the serratus anterior to the upper three ribs are visualized and transected 1 cm from their insertion on each rib using cautery. The insertions of the scalenus medius and posticus and serratus posterior superior are similarly divided from the upper two ribs (Fig. 65-5). Subperiosteal
resection of the third, second, and first ribs is performed in that order, since the first rib is not well visualized until the two lower ribs are resected. Incising the periosteum with a scalpel rather than cautery allows for a cleaner and more rapid stripping of the ribs. The second and third ribs are resected from the transverse processes to the costochondral junction. The first rib requires special care in its management, and the surgeon must be familiar with the neurovascular relationships to the upper surface of the rib (Fig. 65-6). It is best approached by first freeing the lower surface of its periosteum and inserting a finger
in that interval before using the Haight or Matson elevators to elevate the periosteum on its upper surface (Fig. 65-7). The first rib is divided posteriorly 1 cm from the transverse process and retracted downward and outward with a bone-holding forceps (Fig. 65-8). The attachment of the scalenus anterior to the first rib is thus visualized and it is carefully divided, avoiding injury to the subclavian vessels and the brachial plexus. The rib is similarly divided at the costochondral junction. After excision of the ribs, the sacrospinalus muscle is reflected medially and retracted with the Semb retractor. The transverse processes are exposed and, using the Coryllos periosteal elevator, the transverse processes and necks of the ribs—except for the transverse process of the first rib—are denuded and resected using the box-nosed rongeur (Fig. 65-9). Hemostasis is
obtained and the wound is closed in layers with absorbable sutures. If the pleura is opened, intercostal tube drainage is used until the pneumothorax has been evacuated. A bulky compression dressing is applied, taking care to avoid inhibiting respiratory excursions of the opposite hemithorax. The patient is encouraged to lie upon a bolster with the operative side down. Chest and arm physical therapy are used after each operative stage.
scapula (Fig. 65-3). The trapezius, rhomboids, and upper portion of the latissimus dorsi muscles are divided in the line of the incision. If the use of the latissimus as an intrathoracic muscle flap is contemplated, it may be mobilized instead of being incised. The scapula is elevated. A 3-cm incision is made in the periosteum on the upper border of the sixth rib, and this periosteum is elevated, thus allowing a flange of a rib spreader to be inserted (Fig. 65-4). The rib spreader is opened and the upper flange elevates and fixes the scapula, thus freeing the assistant for other duties. The attachments of the serratus anterior to the upper three ribs are visualized and transected 1 cm from their insertion on each rib using cautery. The insertions of the scalenus medius and posticus and serratus posterior superior are similarly divided from the upper two ribs (Fig. 65-5). Subperiosteal
resection of the third, second, and first ribs is performed in that order, since the first rib is not well visualized until the two lower ribs are resected. Incising the periosteum with a scalpel rather than cautery allows for a cleaner and more rapid stripping of the ribs. The second and third ribs are resected from the transverse processes to the costochondral junction. The first rib requires special care in its management, and the surgeon must be familiar with the neurovascular relationships to the upper surface of the rib (Fig. 65-6). It is best approached by first freeing the lower surface of its periosteum and inserting a finger
in that interval before using the Haight or Matson elevators to elevate the periosteum on its upper surface (Fig. 65-7). The first rib is divided posteriorly 1 cm from the transverse process and retracted downward and outward with a bone-holding forceps (Fig. 65-8). The attachment of the scalenus anterior to the first rib is thus visualized and it is carefully divided, avoiding injury to the subclavian vessels and the brachial plexus. The rib is similarly divided at the costochondral junction. After excision of the ribs, the sacrospinalus muscle is reflected medially and retracted with the Semb retractor. The transverse processes are exposed and, using the Coryllos periosteal elevator, the transverse processes and necks of the ribs—except for the transverse process of the first rib—are denuded and resected using the box-nosed rongeur (Fig. 65-9). Hemostasis is
obtained and the wound is closed in layers with absorbable sutures. If the pleura is opened, intercostal tube drainage is used until the pneumothorax has been evacuated. A bulky compression dressing is applied, taking care to avoid inhibiting respiratory excursions of the opposite hemithorax. The patient is encouraged to lie upon a bolster with the operative side down. Chest and arm physical therapy are used after each operative stage.
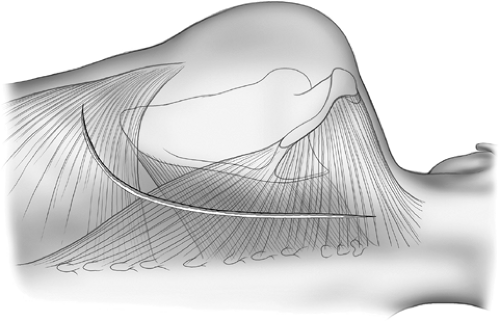
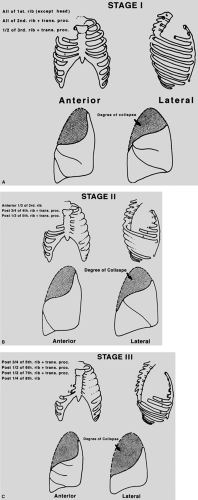 Figure 65-1. Degrees of lung collapse after sequential stages of thoracoplasty for tuberculosis. Degrees of lung collapse after sequential stages of thoracoplasty for tuberculosis. |
In cases of apical cavities located posteriorly in the region of the paravertebral sulcus, it may be necessary to collapse
them using the Semb apicolysis, as described in 1935.27,28 After removing the first rib, the intercostal bundle between the first and second ribs is mobilized and divided, allowing access to the extrafascial plane. The apical parietal pleura is mobilized in this plane, taking care to avoid injury to subclavian vessels and the brachial plexus. This extrapleural tissue is depressed to a level no lower than the fourth vertebral body. Apicolysis is of particular value in the management of empyema reaching to this level, thus obviating the need for first-rib resection.
them using the Semb apicolysis, as described in 1935.27,28 After removing the first rib, the intercostal bundle between the first and second ribs is mobilized and divided, allowing access to the extrafascial plane. The apical parietal pleura is mobilized in this plane, taking care to avoid injury to subclavian vessels and the brachial plexus. This extrapleural tissue is depressed to a level no lower than the fourth vertebral body. Apicolysis is of particular value in the management of empyema reaching to this level, thus obviating the need for first-rib resection.
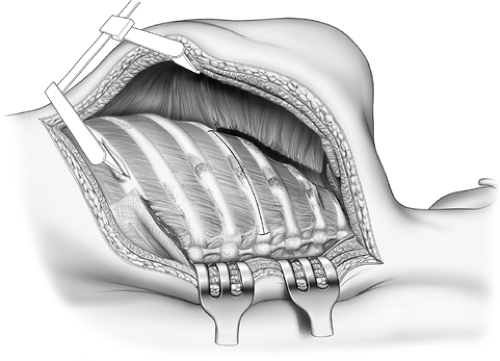
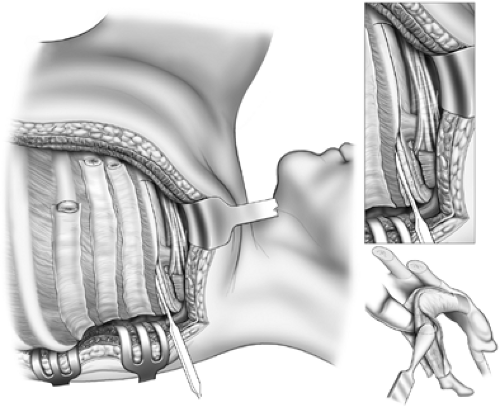
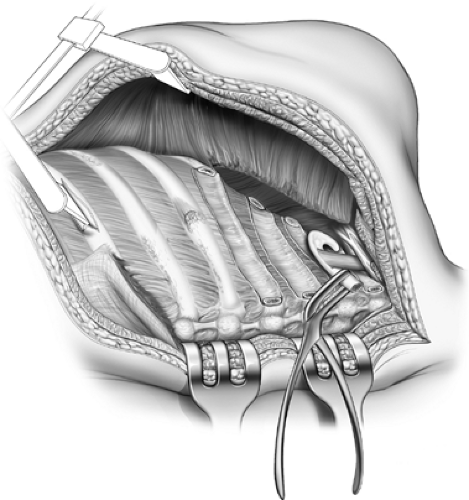 Figure 65-8. The first rib has been divided posteriorly and grasped with sequestrum forceps. The scalenus is transected and the rib excised at its junction with the costal cartilage. |
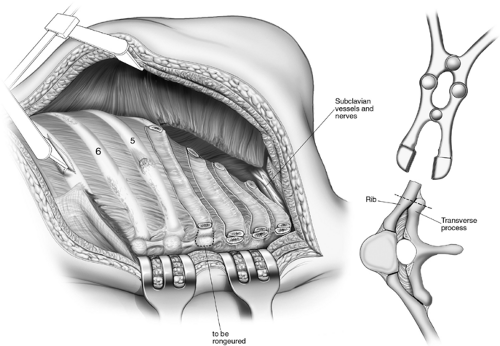 Figure 65-9. Paravertebral muscles reflected and rib necks and transverse processes resected.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|