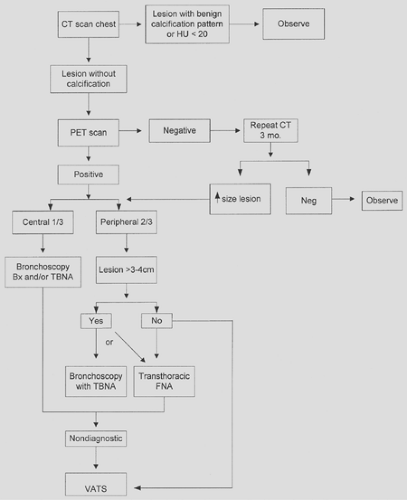
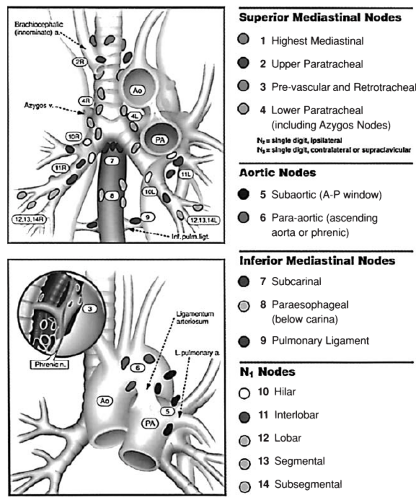
Diagnosis and Staging of Lung Cancer
Carolyn E. Reed
Gerard A. Silvestri
Once the radiographic (see Chapter 108) or clinical presentation (see Chapter 107) raises the possibility of lung cancer, the physician must proceed in an expeditious manner to confirm a diagnosis. The choice of approach will be influenced by lesion characteristics (e.g., size, location), presence or absence of symptoms (e.g., hemoptysis, obstructive pneumonia), and evidence of extrathoracic disease. The evaluation should establish the diagnosis as well as determine the extent or stage of the cancer. This information is critical for treatment decisions and discussion of prognosis with the patient. At times, the diagnosis and stage can be determined by a single test (e.g., liver biopsy, thoracentesis of a pleural effusion). In most cases, careful evaluation of the tumor status (T), nodal status (N), and evidence of metastatic disease (M) requires consideration of a variety of noninvasive and invasive procedures. The staging should be performed in a cost-effective manner.
Diagnosis of Lung Cancer
Sputum Cytology
Since the advent of fiberoptic bronchoscopy, the use of sputum cytology for the diagnosis of lung cancer has declined dramatically. However, this diagnostic technique should be considered in certain cases because it has no risk to the patient and is inexpensive. Sputum cytology is most beneficial when the lesion is central or the patient presents with hemoptysis or when a bronchoscopy or transthoracic needle biopsy poses an undue risk. In a recently published meta-analysis including 30,000 patients, the pooled sensitivity of sputum cytology was 66% with a specificity of 99%.57
Fiberoptic Bronchoscopy
Fiberoptic bronchoscopy is used to diagnose, stage, and, in some cases, treat lung cancer. Diagnostic techniques include endobronchial forceps biopsy, endobronchial brushing, bronchial washing, bronchoalveolar lavage (BAL), and transbronchial needle aspiration (TBNA). Fiberoptic bronchoscopy is the diagnostic procedure of choice for patients with centrally located lung masses.62 Such a mass may be present as an endobronchial exophytic lesion or a bronchial submucosal or infiltrative lesion, or it may cause extrinsic bronchial compression. The diagnostic yield of fiberoptic bronchoscopy for a central bronchogenic carcinoma from 35 pooled series totaling 4,507 patients is 74%.57 Three to four biopsy specimens maximize diagnostic yield. For those where bronchial brush alone is used, the sensitivity drops to 61%. The use of either bronchial wash or TBNA have even lower sensitivities (47% and 56%, respectively) when used alone for central lesions. However, when combined, the sensitivity is quite high at 88%.57
In choosing which diagnostic procedures should be combined, one should assess the lesion. For example, a submucosal lesion is far more likely to be amenable to TBNA than to brush, whereas brush would be better for lesions that infiltrate the airway.57
The use of fiberoptic bronchoscopy for peripheral lesions is dependent on size. For lesions <2 cm, the sensitivity is relatively poor at 34%.57 However, for lesions >2 cm, the sensitivity is an acceptable 63%. When reports of all peripheral lesions (n = 5,742) and some combination of diagnostic tests (biopsy, brush, wash, TBNA) are combined, the sensitivity approaches 80%.57 The complication rate of fiberoptic bronchoscopy is <1% and includes cough, hypoxemia, cardiac arrhythmia, bleeding, pneumothorax, and iatrogenic infection. Death is extremely rare and reported to be less than 0.04% in >68,000 cases.15
Several newer image-guided systems have become available to aid the bronchoscopist in reaching the peripheral solitary pulmonary nodule. The first is the use of radial probe ultrasound to localize the lesion. Reports by Kurimoto and colleagues were able to establish a diagnosis in 77% of patients irrespective of lesion size.32 Still another fascinating technology is electromagnetic navigation, which is like having a GPS for your bronchoscope much like the one you might use for your car. The data for this technology, either alone or in conjunction with endobronchial ultrasound, reveal an increased ability to diagnose peripheral lesions in the 70% range.20,66 Perhaps the best use of these technologies would be in a patient who is not an operative candidate but needs tissue for treatment and is at high risk for pneumothorax.
Transthoracic Fine-Needle Aspiration
Transthoracic fine-needle aspiration (FNA) has been the procedure of choice for the diagnosis of peripheral pulmonary nodules measuring <3 cm in diameter, particularly those lesions lateral to the midclavicular line. FNA biopsy can be performed using fluoroscopic computed tomography (CT) or ultrasonographic guidance. However, CT-guided FNA is the most commonly used modality. For peripheral lung lesions, a recent meta-analysis has revealed a sensitivity of 90% with a specificity approaching 100%.57 The presence of an on-site cytopathologist improves the diagnostic yield. Addition of a core needle biopsy to FNA increases the specificity of obtaining a definitive benign diagnosis. Unfortunately, a negative or nondiagnostic FNA does not reassure the clinician that the patient does not have lung cancer because the false-negative rate is between 20% and 30%. A repeat transthoracic FNA is diagnostic in 35% to 65% of these patients.
The most common complications of transthoracic FNA are bleeding (hemoptysis, hematoma, and hemothorax) and pneumothorax. As reviewed by Savage and colleagues,62 postbiopsy bleeding is usually self-limited, and severe bleeding is seen almost exclusively with core biopsy. Minor hemoptysis occurs in 5% to 10% of cases. The incidence of pneumothorax is dependent on type of biopsy (core versus FNA), depth and size of lesion, number of biopsy attempts, and presence of emphysema, among other factors. While pneumothorax can occur in 25% of patients undergoing needle biopsy, chest tube placement is required in only 5% of patients sustaining a pneumo- thorax.
After nondiagnostic bronchoscopy, the surgeon must decide whether to proceed with transthoracic FNA or with more invasive diagnostic approaches, such as video-assisted thoracic surgery (VATS) or thoracotomy. Swensen and colleagues77 identified three clinical characteristics and three radiologic characteristics that were independent predictors of malignancy (Table 109-1). In patients who have these characteristics, FNA only delays surgery, and a more aggressive approach is warranted. However, there are circumstances in which FNA is recommended to avoid surgery:
The patient is a high operative risk.
The patient has a low risk of malignancy based on clinical and radiologic characteristics.
A definite benign diagnosis is considered likely.
The patient prefers to have a diagnosis of cancer before proceeding to the operating room.
The patient is not an operative candidate but tissue confirmation is needed before definitive treatment with radiation therapy or chemotherapy or both.
Table 109-1 Characteristics of Solitary Pulmonary Nodules Predicting Malignancy | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Video-Assisted Thoracic Surgery
For patients with peripheral indeterminate pulmonary nodules, wedge excision via VATS offers an alternative to transthoracic needle biopsy. When clinical or radiologic (i.e., CT lesion enhancement >20 Hounsfield units or PET-positive) characteristics suggest high likelihood of malignancy, this more invasive diagnostic technique is warranted. Mack and colleagues38 reported the results of 242 patients undergoing thoracoscopic excisional biopsy as the primary diagnostic method for indeterminate solitary pulmonary nodules. Such lesions were defined as <3 cm in diameter, noncalcified, and located in the outer third of the lung parenchyma. Only two patients required thoracotomy to locate the nodules; a definitive diagnosis was obtained in all patients.
Before performing VATS, the CT scan should be carefully examined to determine the likelihood that the nodule can be located at thoracoscopy. If the nodule is pleural-based and >1 cm, visualization of the nodule can be anticipated. If the nodule is immediately subpleural, effacement of the lung parenchyma around the nodule as the lung collapses aids in detection. The increased use of computed tomography (CT) in lung cancer screening programs has led to the identification of numerous subcentimeter nodules. For nodules deeper than 1 cm below the pleural surface or <1 cm in diameter, several techniques can be helpful. The CT scan may indicate subtle pleural puckering, or soft tissue windows may reveal the nodule adjacent to the fissure. A blunt grasping instrument may be used for palpation of the lung and give the surgeon a partial tactile sense. A finger inserted through a port site placed over the lesion, as guided by CT, can palpate a partially inflated lung. Several different nodule localization techniques have been developed to help mark small subpleural lesions for thoracoscopic wedge excision. These include visual markers such as methylene blue and hook wires, fluoroscopic localization using various radiopaque markers, radiotracer localization techniques, and thoracic endosonography.6,41,45,49,51,54,60,73,75,84,87,89
Thoracotomy
When other noninvasive diagnostic techniques have been unsuccessful and the lesion has features suggestive of malignancy, open thoracotomy should be performed. If the lesion is deep within a lobe and cannot be removed easily and completely by a wedge excision, Tru-Cut needle biopsy should be performed.
Staging of Lung Cancer
The staging of lung cancer is critical for planning treatment strategies, defining prognostic subgroups, and comparing research data and the results of clinical trials. Staging provides a common language of communication for physicians caring for the patient. The staging process should be accurate and reproducible.
Over the last two decades the staging system for non-small-cell lung cancer (NSCLC) has undergone significant changes in an attempt to minimize variability of prognosis within each group and correlate different treatment strategies for different groups. Mountain46 has refined the TNM staging system to increase specificity in stage classification and decrease the heterogeneity of end results existing for the TNM categories within stage groups. This system used a database of 5,319 patients with primary lung cancer treated at the M. D. Anderson Cancer Center from 1975 to 1988 or by the North American Lung Cancer Study Group from 1977 to 1982. The TNM descriptors are detailed in Table 109-2.
Mountain and Dresler47 recommended a classification of regional lymph node stations that unified the system developed and reported by Naruke and colleagues48 and the system advocated by the American Thoracic Society and the North American Lung Cancer Study Group. The schema was adopted by the American Joint Committee on Cancer (AJCC) and the Prognostic Factors TNM Committee of the Union Internationale Contre le Cancer at the 1996 annual meeting. The regional lymph node stations are illustrated in Figure 109-2 (see Color Fig. 109-2) and defined in Table 109-2. All N2 nodes are contained within the mediastinal pleural envelope and have single-digit numbers. According to the location of the primary tumor, ipsilateral N2 nodes are designated right or left. Midline prevascular and retrotracheal lymph nodes are considered ipsilateral. All N1 nodes (numbered 10 through 14) lie distal to the mediastinal pleural reflection and are within the visceral pleura. Stage grouping in Mountain’s revised system is shown in Table 109-3 and Figure 109-3 (see Color Fig. 109-3).
Because of problems that have arisen in the current staging system, the International Association for the Study of Lung Cancer (IASLC) has assembled a massive dataset including >100,000 entries from 23 institutions in 12 countries in Europe, the United States, and Australia.36,23 The main suggestions are in the T and M classification, with N status remaining the same. It was found that tumor size was an important prognosticator and therefore recommend that the T factor be subdivided based on five different size criteria. Because survivorship was better, they recommend reclassification of primary tumors with satellite
nodules in the same lobe from T4 to T3 and that additional nodules in a different lobe of the ipsilateral lung be moved from an M1 designation to T4. Malignant pleural effusion is currently classified as T4, or “wet” IIIB disease, despite the fact that the survival of patients in this group is much more like that of those with metastatic rather than locally advanced disease. The IASLC appropriately proposes moving these patients to M1. One final recommendation is that the M status be split into M1a (metastatic disease confined to the chest) and M1b (extrathoracic metastatic disease), based on the finding that survival is better in those with metastatic disease confined to the thorax.23,70
nodules in the same lobe from T4 to T3 and that additional nodules in a different lobe of the ipsilateral lung be moved from an M1 designation to T4. Malignant pleural effusion is currently classified as T4, or “wet” IIIB disease, despite the fact that the survival of patients in this group is much more like that of those with metastatic rather than locally advanced disease. The IASLC appropriately proposes moving these patients to M1. One final recommendation is that the M status be split into M1a (metastatic disease confined to the chest) and M1b (extrathoracic metastatic disease), based on the finding that survival is better in those with metastatic disease confined to the thorax.23,70
Table 109-2 TNM Definitions | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
The Noninvasive Staging of Lung Cancer
Computed Tomography for Intrathoracic Staging
CT of the chest is the most widely used imaging modality for staging lung cancer. A CT scan is almost always obtained after an abnormality is discovered on plain chest radiography. There are clinical scenarios for which chest radiography alone may suffice (e.g., multiple metastatic pulmonary nodules in a patient with a poor performance status or gross rib destruction with chest wall invasion). However, because plain chest radiography is not sensitive enough to detect mediastinal lymphadenopathy or invasion of the chest wall or pleura, CT of the chest is almost always necessary.
For evaluation of the primary tumor, CT cannot readily distinguish between T3/T4 tumors and T1/T2 tumors. In fact, in a study by Webb and colleagues,85 CT was able to discriminate between advanced chest wall tumors and primary tumors in only 62% of the cases. When rib destruction was present or there was a definite chest wall mass, CT had a better predicted value. The surgeon cannot rely solely on CT for central tumors that may invade the mediastinum. The sensitivity of CT for invasion of the mediastinum is low, in the range of 60% to 75%. Occasionally, invasion of a vessel can be detected by CT. Given this low sensitivity, there are occasions when surgical exploration is the only way to definitively stage for tumor invasion into the mediastinum (T4 disease). It was initially thought that magnetic resonance imaging (MRI) would be useful in detecting mediastinal and chest wall invasion. However, the sensitivity and specificity of this technique are not significantly higher than those of CT alone. MRI is useful in evaluating superior sulcus (Pancoast) tumors for involvement of the brachial plexus, spinal cord, chest wall, and subclavian artery.
The use of CT to detect mediastinal lymphadenopathy is fraught with difficulties. Although it is an excellent tool for detecting enlarged lymph nodes, it cannot differentiate benignity from malignancy. Lymph nodes in the mediastinum are considered enlarged if they are >1 cm in short-axis diameter. The finding of an enlarged lymph node in the mediastinum in patients with a known primary lung cancer does not ensure that those lymph nodes will have metastatic deposits. False-positive lymph nodes are especially common in patients with postobstructive pneumonia secondary to their primary lung cancer. Patients with other underlying diseases, such as sarcoidosis, may have abnormally enlarged mediastinal lymph nodes. When lymph node enlargement is detected by CT, the onus is on the clinician to prove that the lymph node has a metastatic deposit. It must be emphasized that enlarged lymph nodes on CT alone should not preclude a potentially curative resection. In addition, CT of the chest can be helpful in directing the clinician to the most appropriate staging procedure for lymph node biopsy.
Recently, the American College of Chest Physicians completed an evidence-based review of the staging characteristics of CT for staging the mediastinum in patients with NSCLC; it was initially reported in 2003.71,79 An analysis including 5,111 evaluable patients reported that CT had a pooled sensitivity and specificity of 51% (95% confidence interval [CI], 47%–54%) and 86% (95% CI, 84%–88%), respectively. Thus, 14% of enlarged mediastinal lymph nodes detected on CT will be secondary to benign causes, reinforcing the need to sample these nodes prior to excluding a patient from potentially curative surgery.
There is some controversy regarding whether an invasive mediastinal staging study (i.e., mediastinoscopy) should be performed prior to surgery in patients with no evidence of mediastinal lymphadenopathy by CT. The false-negative rate in patients with peripheral lesions and a radiographically normal CT of the mediastinum is approximately 10% and varies by tumor size.13 Thus, invasive staging of the mediastinum is not recommended for peripheral T1 lesions and normal-sized mediastinal lymph nodes. However, in a clinical scenario where a normal-sized node has uptake on PET, one should proceed to invasive staging to perhaps avoid a futile thoracotomy.
Table 109-3 Revised Stage Grouping for Lung Cancer | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Positron Emission Tomography for Staging Lung Cancer
Perhaps the most significant advance in the staging of lung cancer over the last several years is the use of positron emission tomography (PET). This imaging modality takes advantage of the biological activity of tumor cells. Cancer cells have an increased cellular uptake of glucose and a higher rate of glycolysis than normal cells. The radiolabeled glucose analog fluorodeoxyglucose (FDG) undergoes the same cellular uptake as glucose, but after phosphorylation is not further metabolized and is thus trapped in cells. Accumulation of this isotope can then be detected under a PET camera. The specific criteria for an abnormal PET scan include either a standard uptake value (SUV) of >2.5
or uptake in a lesion that is greater than the background uptake in the mediastinum. PET scans, therefore, provide the clinician with information on the functional activity of a lesion rather than the strictly anatomic information that CT can provide. The two imaging modalities are complementary, and one cannot necessarily replace the other. Integrated PET-CT scanners are now available and studies suggest that they are better than either technology alone or when the studies are performed on separate machines and read together.35 The explosion of research on the utility of PET for the diagnosis and staging of lung cancer can be divided into three categories: locating the solitary pulmonary nodule or mass (PET for nodules and masses is covered in Chapter 96, on the solitary pulmonary nodule), staging the mediastinum, and searching for occult metastatic disease. These are arbitrary distinctions; but when a PET scan is performed for patients with known or suspected lung cancer, information is gathered for all areas (excluding the brain) and clinical decisions are made with the totality of information. However, what has emerged since the last iteration of this book is that some of the luster has worn off of PET; with more data available. The sensitivity, specificity, and accuracy has decreased.
or uptake in a lesion that is greater than the background uptake in the mediastinum. PET scans, therefore, provide the clinician with information on the functional activity of a lesion rather than the strictly anatomic information that CT can provide. The two imaging modalities are complementary, and one cannot necessarily replace the other. Integrated PET-CT scanners are now available and studies suggest that they are better than either technology alone or when the studies are performed on separate machines and read together.35 The explosion of research on the utility of PET for the diagnosis and staging of lung cancer can be divided into three categories: locating the solitary pulmonary nodule or mass (PET for nodules and masses is covered in Chapter 96, on the solitary pulmonary nodule), staging the mediastinum, and searching for occult metastatic disease. These are arbitrary distinctions; but when a PET scan is performed for patients with known or suspected lung cancer, information is gathered for all areas (excluding the brain) and clinical decisions are made with the totality of information. However, what has emerged since the last iteration of this book is that some of the luster has worn off of PET; with more data available. The sensitivity, specificity, and accuracy has decreased.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree