Thoracoabdominal Aortic Aneurysm
DONALD G. HARRIS and ROBERT S. CRAWFORD
Presentation
A 65-year-old woman with hypertension and a 30-pack-year smoking history presented to the emergency department with acute chest pain, dyspnea, nausea, and diaphoresis. The pain was sharp and radiated to her back and was associated with EKG changes and a mildly elevated troponin. An emergent left heart catheterization was negative for coronary artery disease. A chest x-ray showed a widened aortic silhouette.
Differential Diagnosis
Acute chest pain mandates evaluation for cardiac ischemia, and given this patient’s history and high-risk findings, a cardiac evaluation was warranted. However, for any patient with new chest pain, acute aortic syndrome remains in the differential, especially if back pain or abdominal symptoms are present. Acute aortic events include dissection, intramural hematoma, penetrating ulcer, and aneurysm instability or rupture, all of which can be readily diagnosed by CT. Other conditions that should be considered and ruled out include pulmonary embolism, pneumothorax, and esophageal perforation.
Most thoracoabdominal aneurysms (TAAAs) are asymptomatic and are diagnosed incidentally. Alternatively, TAAA expansion with compression of neighboring structures may result in nonspecific complaints including back pain, hoarseness, or respiratory symptoms without causing acute aortic syndrome. These symptoms may mimic a variety of cardiopulmonary or abdominal conditions, such as musculoskeletal disease, pancreatitis, or nephrolithiasis. Because these conditions are relatively more common, even if TAAA is confirmed radiographically, they should be excluded by routine testing.
Workup
TAAAs can be readily diagnosed and classified by CT. To completely define the extent of the aneurysm and the anatomy of the carotid, visceral, and iliofemoral vasculature, a CT of the neck, chest, abdomen, and pelvis with contrast arteriography should be obtained. Because patients often have comorbid chronic kidney dysfunction, IV hydration should be given with contrast studies to prevent nephropathy. Alternatively, magnetic resonance aortography is an option if contrast and radiation exposures are of concern. Once defined, the proximal and distal extent of the lesion dictates how the aneurysm is resected and reconstructed, and the aneurysm can be classified according to the Crawford scheme (Fig. 1).
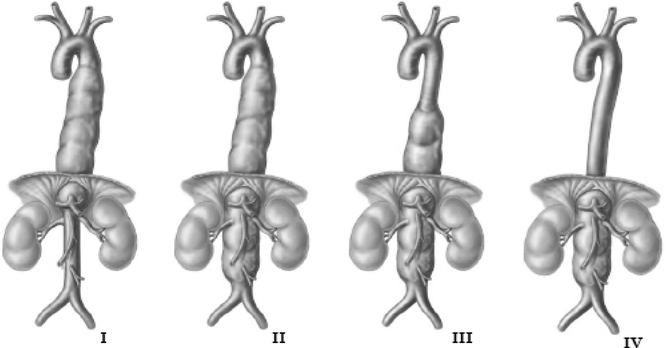
FIGURE 1 Crawford classification of thoracoabdominal aneurysms. (From Cambria RP, Crawford RS. Thoracoabdominal aortic aneurysm repair. In: Jones DB, Pomposelli FB, Upchurch GR, eds. Fischer’s Mastery of Surgery. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:2203, Chapter 217.)
Most TAAAs are due to degenerative aortic disease, which is often associated with hypertension, atherosclerosis, and smoking. As such, patients considered for elective surgery should undergo cardiac risk stratification and pulmonary function testing, with appropriate management as indicated.
Discussion
This patient had a negative cardiac catheterization, but a chest x-ray was suggestive of aortic disease. As such, a contrast CT was obtained and demonstrated diffuse, multilevel aortic degeneration consistent with a Crawford type II aneurysm (Fig. 2). As part of her preoperative cardiac evaluation, a transthoracic echocardiogram was performed, which demonstrated normal left ventricular function and an ejection fraction of 65%.
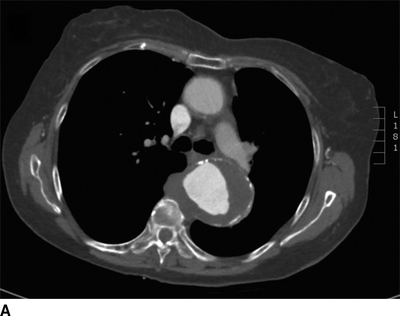
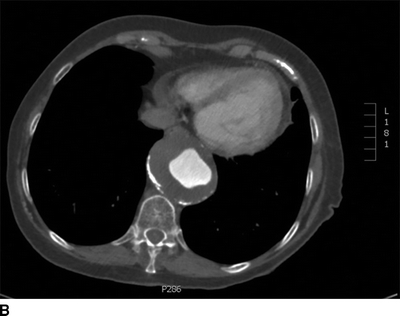
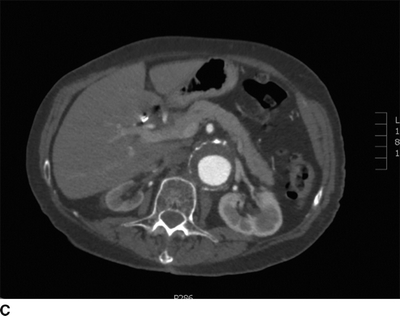
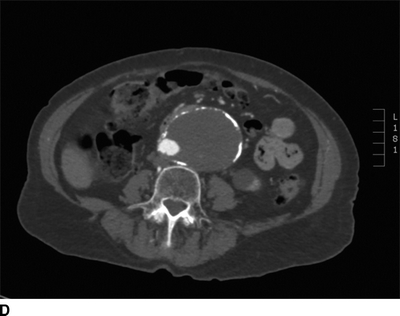
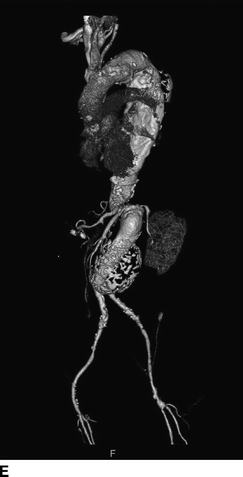
FIGURE 2 A–D:A contrast-enhanced CT of the chest, abdomen, and pelvis demonstrates multilevel aneurysmal degeneration consistent with a Crawford type II TAAA. E: 3D reconstruction of the same aneurysm, viewed from a left anterolateral perspective.
Diagnosis and Treatment
This patient has a symptomatic Crawford type II TAAA complicated by acute aortic syndrome. Her cardiac workup demonstrates acceptable risk for surgery, but the urgent nature of her presentation precludes pulmonary preconditioning and smoking cessation. For asymptomatic patients, repair should be performed if the aneurysm diameter is ≥6 cm (or ≥5 cm with Marfan’s disease) or increases by ≥1.0 cm/year. The patient’s large, symptomatic TAAA and acceptable cardiac risk warrant operative repair to prevent progression to frank rupture.
Surgical Approach
Repair techniques have evolved over time. Although a major advance at the time, the “clamp-and-sew” technique is associated with significant mortality and paraplegia rates, despite improvements in technique and perioperative care. Clamp and sew remains appropriate for type IV lesions where proximal aortic clamping poses decreased risk for spinal ischemia.
More recently, segmental clamping with distal perfusion has become the standard of care for type I to III lesions. Using this method, the aneurysm is clamped, resected, and sequentially reconstructed proximally to distally. Distal arterial perfusion is maintained via full cardiopulmonary bypass, left atriofemoral bypass, or a temporary axillofemoral bypass, which maintains visceral and pelvic perfusion, improves spinal blood flow, and reduces complication rates. The addition of cerebrospinal fluid (CSF) drainage further minimizes the risk of spinal cord ischemia. Finally, intraoperative motor evoked potentials enable monitoring for neurologic deficits and guide reimplantation of intercostal and lumbar arteries if needed.
The details of aortic resection and reconstruction vary depending on the TAAA extent. Generally, a CSF drainage catheter is placed in the lumbar spine subarachnoid space prior to surgery, and right-sided radial and femoral arterial catheters enable monitoring of systemic and distal perfusion, respectively. The patient is placed in a right lateral decubitus position. To facilitate maximal thoracic and visceral exposure, left shoulder is flexed with the arm placed across the table, and the right anterior superior iliac spine is positioned at the break of the table. Appropriate padding is required to prevent skin breakdown at pressure points during these long procedures.
The level of the initial left thoracoabdominal incision is dictated by the proximal aneurysm extent. For type I and II aneurysms, incision through the fourth to sixth intercostal space facilitates proximal and distal exposure, while for type III lesions, a lower incision may suffice. The standard incision for a type IV repair is a thoracoabdominal incision through the ninth interspace. The incision is carried distally along the lateral rectus border to limit functional impairment from rectus transection. Medial visceral rotation is performed to expose the abdominal aorta, which is then dissected inferiorly and superiorly from the left renal artery origin. The left diaphragm is divided circumferentially to spare the phrenic nerve and avoid postoperative paralysis of the hemidiaphragm.
After exposure of the aneurysm, a Dacron graft is sized to the patient’s anatomy and customized for the specific reconstruction. For distal perfusion using left atriofemoral bypass, a circuit is established by cannulating the left atrium via the left inferior pulmonary vein and bypassing blood to the left common femoral artery. Alternatively, a temporary right axillo-femoral bypass can provide distal perfusion. Next, the thoracic aorta is clamped proximal to aneurysm. Retrograde distal aortic perfusion is then established and distal pressure confirmed through a right femoral arterial catheter. The aneurysm is opened, and bleeding intercostal arteries are ligated or occluded with Fogarty balloon catheters for possible later reimplantation.
The proximal anastomosis is performed. Depending on the quality of the proximal aorta, pledgets can be fashioned to provide a secure proximal suture line. The clamps are moved distally and the aorta opened through the visceral segment. The renal arteries are perfused with cold perfusate to limit warm ischemia. The anastomosis of the visceral patch that includes the celiac, superior mesenteric, and right renal arteries is performed in a circumferential fashion. Special attention is paid to the posterior suture line as bleeding from this segment can be difficult to control. The left renal artery is reconstructed separately using an end-to-end PTFE graft. This is fashioned with a gentle curve from the aortic prosthesis to avoid kinking. Alternatively, the renal artery can be reimplanted directly onto the Dacron graft with a Carrel patch. If motor evoked potentials dictate, spinal arteries can be reimplanted.
Finally, the distal aortic anastomosis is performed in an end-to-end fashion. For type I repairs, the anastomosis can be beveled to include the visceral vessels. Perfusion of the legs and visceral arteries is confirmed by palpation and Doppler ultrasound. The aneurysm sac is closed over the graft, the diaphragm is reconstructed, and a left chest tube is placed. Complete hemostasis is ensured, the incision is closed, and the patient is transferred to the intensive care unit.
Potential Pitfalls
Due to frequently comorbid tobacco use and lung disease, these patients are at significant risk for postoperative pulmonary complications. Hemidiaphragm paralysis can be avoided by circumferential, as opposed to radial, division during exposure. This reduces postoperative lung collapse and its associated complications. If there is an inflammatory component to the aneurysm, dissection of the thoracic aorta may be complicated by adhesions to the lung or other mediastinal structures. In this situation, adherence to meticulous technique with sharp dissection minimizes bleeding from the lung parenchyma. If intraoperative evoked motor potential signals indicate spinal ischemia, the critical T9-L1 arteries can be reconstructed as a patch, or individual segments reimplanted as indicated. Because residual aortic tissue can continue to undergo aneurysmal degeneration, the size of the visceral patch should be kept to a minimum. Finally, a potential cause of major postoperative bleeding is a missed patent intercostal or lumbar artery, which should be identified and either ligated or reimplanted (Table 1).
TABLE 1. Open Thoracoabdominal Aortic Aneurysm Repair




